��Ŀ����
8����1���������� 10 �����ʵ������գ���O2��H2��NH4NO3��K2O2��Ba��OH��2��CH4��CO2��NaF��NH3��I2�������Ӽ����зǼ��Լ����Ǣܣ��������Ӽ����м��Լ����Ǣۢݣ�
��2��X��Y ��������Ԫ�����γ� XY2 �ͻ������֪ XY2 �й��� 38 �����ӣ��� XY2Ϊ����Ԫ���γɵ����ӻ���������ʽΪ��
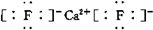 ���� XY2��ṹʽΪ��S=C=S��Ϊ���ۻ�����ʱ��
���� XY2��ṹʽΪ��S=C=S��Ϊ���ۻ�����ʱ����3���Ȼ������������ʷdz����⣬�磺�Ȼ������۵�Ϊ 190�棨2.02��103Pa�������� 180��Ϳ�ʼ�������ݴ��жϣ��Ȼ����ǹ��ۻ��������ۻ���������ӻ������������֤������ж���ȷ��ʵ������������״̬�²��ܵ��磮
���� ��1��һ����˵�����ý�����ǽ����γ����Ӽ����ǽ���֮���γɹ��ۼ���ͬ�ַǽ����γɷǼ��Լ�����ͬ�ǽ����γɼ��Լ���
��2��X��Y��������Ԫ�����γ�XY2�ͻ������֪XY2�й���38�����ӣ���XY2Ϊ����Ԫ���γɵ����ӻ����ΪCaF2����XY2Ϊ���ۻ�����ʱ��ΪCS2��
��3���Ȼ������۵�Ϊ190�棨2.02��103 Pa��������180��Ϳ�ʼ��������֪�۷е�ͣ�����Ӿ�����������ƣ�
��� �⣺��1����O2��ֻ�����ۼ���Ϊ���ʣ�
��H2��ֻ�����ۼ���Ϊ���ʣ�
��NH4NO3�к����Ӽ��ͼ��Լ���Ϊ���ӻ����
��K2O2�к����Ӽ��ͷǼ��Լ���Ϊ���ӻ����
��Ba��OH��2�к����Ӽ��ͼ��Լ���Ϊ���ӻ����
��CH4�к����Լ���Ϊ���ۻ����
��CO2�к����Լ���Ϊ���ۻ����
��NaF��ֻ�����Ӽ���Ϊ���ӻ����
��NH3�к����Լ���Ϊ���ۻ����
��I2��ֻ�����ۼ���Ϊ���ʣ�
��������Ӽ����зǼ��Լ����Ǣܣ��������Ӽ����м��Լ����Ǣۢݣ��ʴ�Ϊ���ܣ��ۢݣ�
��2��X��Y��������Ԫ�����γ�XY2�ͻ������֪XY2�й���38�����ӣ���XY2Ϊ����Ԫ���γɵ����ӻ����ΪCaF2�������ʽΪ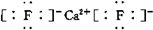 ����XY2Ϊ���ۻ�����ʱ��ΪCS2����ṹʽΪS=C=S���ʴ�Ϊ��
����XY2Ϊ���ۻ�����ʱ��ΪCS2����ṹʽΪS=C=S���ʴ�Ϊ��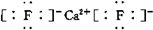 ��S=C=S��
��S=C=S��
��3���Ȼ������۵�Ϊ190�棨2.02��103 Pa��������180��Ϳ�ʼ��������֪�۷е�ͣ�����Ӿ�����������ƣ���֪�ɹ��ۼ��γɵĹ��ۻ��������������״̬�²��ܵ�����ʵ��֤����
�ʴ�Ϊ�����ۻ��������״̬�²��ܵ��磮
���� ���⿼��λ�á��ṹ�����ʵĹ�ϵ��Ϊ��Ƶ���㣬����Ԫ�ص�λ�á�Ԫ�ػ���������Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע��Ԫ�������ɵ�Ӧ�ã���Ŀ�ѶȲ���

| A�� | ������Է�������Ϊ���� | |
| B�� | �������������Է���������ż�� | |
| C�� | �������ĺ������������Է�������Ϊż�� | |
| D�� | �ӡ�ȩ�������ᡢ������Է�������Ϊ���� |
��1����PM2.5����������ˮ�����Ƴɴ���������
����ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�������
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ��/mol•L-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
��2��Ϊ����SO2���ŷţ�����ȡ�Ĵ�ʩ�У��ٽ�úת��Ϊ�������ȼ�ϣ�
��֪��H2��g��+$\frac{1}{2}$O2��g���TH2O��g����H=-241.8kJ•mol-1
C��s��+$\frac{1}{2}$O2��g���TCO��g����H=-110.5kJ•mol-1
д����̿��ˮ������Ӧ���Ȼ�ѧ����ʽ��C��s��+H2O��g���TCO��g��+H2��g����H=+131.3kJ•mol-1��
��ϴ�Ӻ�SO2���������������ʿ���ϴ�Ӽ�����a��b��
a��Ca��OH��2 b��Na2CO3 c��CaCl2 d��NaHS��
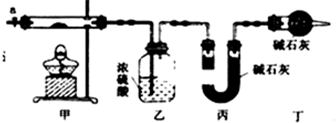
��1������ͼ��ʾװ����װ�������������װ�������ԣ���ȡ3.65g��Ʒ����Ӳ�ʲ������ڣ����ȣ�����װ���в��������ݲ���ʱ��װ���У���ʵ������ֹͣ���ȣ�����a������ͨ����������ӣ�ͨ�������Ŀ�������ɵ�CO2��H2O��g��ȫ�������ҡ���װ�ã�
��2��ijͬѧ��Ϊ����ʵ���д���һ��ȱ�ݣ���ȱ����δ���ǿ����е�ˮ�����Ͷ�����̼��ʵ���Ӱ�죮
��3��ͨ����ȷʵ�����ҡ���װ�����طֱ�Ϊ0.36g��0.88g����ü�ʽ̼���ܵĻ�ѧʽΪCo3��OH��4��CO3��2��
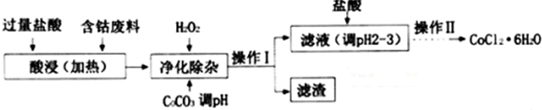
��4��CoCl2•6H2O���������ˮ������Ӽ����Ժ��ܷ��ϣ�������Fe��Al�����ʣ���ȡCoCl2•6H2O��һ�ֹ������£�
��֪��25��ʱ
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 |
| ��ʼ������pH | 2.3 | 7.5 | 7.6 | 3.4 |
| ��ȫ������pH | 4.1 | 9.7 | 9.2 | 5.2 |
�ڼ���CoCO3��pHΪ5.2��7.6�������I��õ������ɷ�ΪFe��OH��3��Al��OH��3��
�ۼ��������pHΪ2��3��Ŀ��Ϊ����CoCl2��ˮ�⣮
��CH3��CH2��2CH3
��CH3��CH2��3CH3
�ۣ�CH3��3CH
�ܣ�CH3��2CHCH2CH3��
| A�� | �ܢڢ٢� | B�� | �ڢܢ٢� | C�� | �ܢ٢ڢ� | D�� | �ڢܢۢ� |
 ����ȷ����Ϊ��������
����ȷ����Ϊ��������| A�� | 3-��4��4-���һ����� | B�� | 3-��3��4-���һ����� | ||
| C�� | 4-��2��2-���һ����� | D�� | 3��4-����3-�һ����� |
| A�� | CH3CH2OCH3 | B�� | CH3CH��OH��CH3 | C�� | CH3CH2 CH2CH2OH | D�� | CH3CH2CHO |
�ټ���AgNO3��Һ �ڼ���NaOH��Һ���� �ۼ�������HNO3 ����ȴ��
| A�� | �٢ڢܢ� | B�� | �ڢܢۢ� | C�� | �ڢܢ� | D�� | �ۢܢ� |
 ��ⷨ��������������������ɻ������ᣬ���нϸߵĻ���Ч��;���Ч�森ʵ����ģ���ⷨ����NOx��װ����ͼ��ʾ��ͼ�е缫��Ϊʯī�缫����
��ⷨ��������������������ɻ������ᣬ���нϸߵĻ���Ч��;���Ч�森ʵ����ģ���ⷨ����NOx��װ����ͼ��ʾ��ͼ�е缫��Ϊʯī�缫����