��Ŀ����
����ϩ�Ľṹʽ ���ڿ�����ȼ�յ����� ���÷�Ӧ�������� �������У����˶�Ա������˻�Ť��ʱ����ҽ�漴����Ա�����˲�λ����ҩ�������飨�е�12.27�棩�����оֲ��䶳����Ӧ��������д����ϩ���Ȼ����Ƶ�������Ļ�ѧ��Ӧ����ʽ ������Һ�巴Ӧ���屽���÷�Ӧ��ѧ����ʽ �������õ��屽�г������嵥�ʣ���ȥ��������Լ��� ���÷�Ӧ��ѧ����ʽ ��
��ij����ij����һ��˫����ϩ���Ļ�����壬��ͬ��ͬѹ�¶�����������ܶ�Ϊ13��ȡ�˻������4.48L����״���£�ͨ��������ˮ�У���ˮ����2.8g�����ϩ����Ħ������
����ϩ�����������干a mol����b mol������������һ�ܱ������У���ȼ���ַ�Ӧ����ϩ������ȫ�������꣬�õ�CO��CO2�Ļ�������45gˮ������
��1����a=1ʱ����ϩ����������ʵ���֮��n��C2H4����n��C2H6��= ��
��2����a=1ʱ���ҷ�Ӧ��CO��CO2�����������ʵ���Ϊ��Ӧǰ������2/3ʱ����b= ���õ���CO��CO2�����ʵ���֮��n��CO����n��CO2��= ��
��3��a��ȡֵ��Χ�� ��
��ij����ij����һ��˫����ϩ���Ļ�����壬��ͬ��ͬѹ�¶�����������ܶ�Ϊ13��ȡ�˻������4.48L����״���£�ͨ��������ˮ�У���ˮ����2.8g�����ϩ����Ħ������
����ϩ�����������干a mol����b mol������������һ�ܱ������У���ȼ���ַ�Ӧ����ϩ������ȫ�������꣬�õ�CO��CO2�Ļ�������45gˮ������
��1����a=1ʱ����ϩ����������ʵ���֮��n��C2H4����n��C2H6��=
��2����a=1ʱ���ҷ�Ӧ��CO��CO2�����������ʵ���Ϊ��Ӧǰ������2/3ʱ����b=
��3��a��ȡֵ��Χ��
���㣺��ϩ�Ļ�ѧ����,��������,�йػ���ﷴӦ�ļ���,�й��л������ʽȷ���ļ���
ר�⣺������,�л���Ӧ
������������ϩ�ĵ���ʽд����ϩ�Ľṹʽ��������ϩ��̼���ϸ߷���ȼ�յ�������ϩ���Ȼ�����һ�������·����ӳɷ�Ӧ���������飬�ӳɷ�Ӧ��ԭ��������Ϊ100%������ԭ�ӱ���ԭ��ȡ�������屽���廯�⣻���ݳ���������Ҫ���������������ټ�����Լ�ֻ�������ʷ�Ӧ��������ԭ���ʷ�Ӧ���ڷ�Ӧ���������µ����ʣ��Դ�������ȥ�屽�е��壻
����ͬ�����£��ܶ�֮�ȵ�����Է�������֮�ȣ��ݴ˼������������Է���������
��1������ϩ����������ʵ����ֱ�Ϊxmol��ymol�����ݶ������ʵ���֮�������ɵ�ˮ��Hԭ���غ��з��̣��ݴ˼�����
��2������̼Ԫ���غ���㷴Ӧ��CO��CO2�����������ʵ���֮�ͣ��ݴ˼���b��ֵ��
�Ӧ��CO��CO2�����ʵ����ֱ�Ϊamol��bmol�����ݶ���֮������ԭ���غ��з��̼��㣻
��3������Hԭ���غ����ü������ֻ����ϩʱ��aֵ���ֻ������ʱ��aֵ��С��
����ͬ�����£��ܶ�֮�ȵ�����Է�������֮�ȣ��ݴ˼������������Է���������
��1������ϩ����������ʵ����ֱ�Ϊxmol��ymol�����ݶ������ʵ���֮�������ɵ�ˮ��Hԭ���غ��з��̣��ݴ˼�����
��2������̼Ԫ���غ���㷴Ӧ��CO��CO2�����������ʵ���֮�ͣ��ݴ˼���b��ֵ��
�Ӧ��CO��CO2�����ʵ����ֱ�Ϊamol��bmol�����ݶ���֮������ԭ���غ��з��̼��㣻
��3������Hԭ���غ����ü������ֻ����ϩʱ��aֵ���ֻ������ʱ��aֵ��С��
���
�⣺����ϩ�ĽṹʽΪ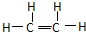 ����ϩ��̼���ϸߣ�ȼ�շ���������ӦC2H4+3O2
����ϩ��̼���ϸߣ�ȼ�շ���������ӦC2H4+3O2
2CO2+2H2O����������̣���ϩ���Ȼ�����һ�������·����ӳɷ�Ӧ���������飬����ʽΪCH2=CH2+HCl��CH3CH2Cl������Һ���ڴ��������·�Ӧ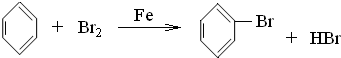 �����屽���÷�ӦΪȡ����Ӧ���������ƿ��Ժ��嵥�ʷ�ӦBr2+2NaOH�TNaBr+NaBrO+H2O�����廯�ơ��������Ƶ�ˮ��Һ�����屽�����ܣ������ٷ�Һ�����룻
�����屽���÷�ӦΪȡ����Ӧ���������ƿ��Ժ��嵥�ʷ�ӦBr2+2NaOH�TNaBr+NaBrO+H2O�����廯�ơ��������Ƶ�ˮ��Һ�����屽�����ܣ������ٷ�Һ�����룻
�ʴ�Ϊ��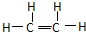 ���к��̣�������Ӧ��CH2=CH2+HCl��CH3CH2Cl��
���к��̣�������Ӧ��CH2=CH2+HCl��CH3CH2Cl��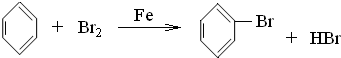 ���������ƣ�Br2+2NaOH�TNaBr+NaBrO+H2O��
���������ƣ�Br2+2NaOH�TNaBr+NaBrO+H2O��
����ͬ��ͬѹ�£�������������������ܶ�Ϊ13���ʻ�������ƽ����Է�������Ϊ13��2=26����ϩ������Է���������СΪ28��C2H4����С��26��һ��Ϊ������������һ��ΪCH4�������4.48L�����������ʵ���Ϊ��n=
=
=0.2mol���ʻ�������������Ϊ0.2mol��26g/mol=5.2g����ˮ����2.8g��ӦΪϩ��������������n��CH4��=
=0.15mol��ϩ�������ʵ���Ϊ0.2mol-0.15mol=0.05mol����ϩ������Է�������ΪM=
=
=56g/mol��
�ʴ�Ϊ��56g/mol��
��1������ϩ����������ʵ����ֱ�Ϊxmol��ymol�����ݶ������ʵ���֮����Hԭ���غ��з��̣���x+y=1��4x+6y=
����ã�x=0.5��y=0.5��
��n��C2H4����n��C2H6��=0.5mol��0.5mol=1��1��
�ʴ�Ϊ��1��1��
��2������̼Ԫ���غ��֪����Ӧ��CO��CO2�����������ʵ���֮��Ϊ1mol��2=2mol����
b=2�����b=3��
�Ӧ��CO��CO2�����ʵ����ֱ�Ϊamol��bmol�����ݶ���֮������ԭ���غ��з��̣���a+b=2��a+2b=3��2-
����ã�a=0.5��b=1.5����n��CO����n��CO2��=0.5mol��1.5mol=1��3��
�ʴ�Ϊ��3��1��3��
��3������ˮ�����ʵ���=
=2.5molֻ����ϩʱ��aֵ�����Hԭ���غ��֪��a�ļ���ֵΪ
=
mol��ֻ������ʱ��aֵ��С������Hԭ���غ��֪��a�ļ�СֵΪ
=
mol����a��ȡֵ��Χ��
��a��
��
�ʴ�Ϊ��
��a��
��
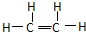 ����ϩ��̼���ϸߣ�ȼ�շ���������ӦC2H4+3O2
����ϩ��̼���ϸߣ�ȼ�շ���������ӦC2H4+3O2
| ||
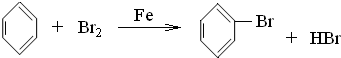 �����屽���÷�ӦΪȡ����Ӧ���������ƿ��Ժ��嵥�ʷ�ӦBr2+2NaOH�TNaBr+NaBrO+H2O�����廯�ơ��������Ƶ�ˮ��Һ�����屽�����ܣ������ٷ�Һ�����룻
�����屽���÷�ӦΪȡ����Ӧ���������ƿ��Ժ��嵥�ʷ�ӦBr2+2NaOH�TNaBr+NaBrO+H2O�����廯�ơ��������Ƶ�ˮ��Һ�����屽�����ܣ������ٷ�Һ�����룻�ʴ�Ϊ��
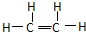 ���к��̣�������Ӧ��CH2=CH2+HCl��CH3CH2Cl��
���к��̣�������Ӧ��CH2=CH2+HCl��CH3CH2Cl��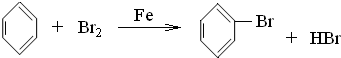 ���������ƣ�Br2+2NaOH�TNaBr+NaBrO+H2O��
���������ƣ�Br2+2NaOH�TNaBr+NaBrO+H2O������ͬ��ͬѹ�£�������������������ܶ�Ϊ13���ʻ�������ƽ����Է�������Ϊ13��2=26����ϩ������Է���������СΪ28��C2H4����С��26��һ��Ϊ������������һ��ΪCH4�������4.48L�����������ʵ���Ϊ��n=
| v |
| vm |
| 4.48L |
| 22.4mol/L |
| 5.2g-2.8g |
| 16g/mol |
| m |
| n |
| 2.8g |
| 0.05mol |
�ʴ�Ϊ��56g/mol��
��1������ϩ����������ʵ����ֱ�Ϊxmol��ymol�����ݶ������ʵ���֮����Hԭ���غ��з��̣���x+y=1��4x+6y=
| 45 |
| 18 |
��n��C2H4����n��C2H6��=0.5mol��0.5mol=1��1��
�ʴ�Ϊ��1��1��
��2������̼Ԫ���غ��֪����Ӧ��CO��CO2�����������ʵ���֮��Ϊ1mol��2=2mol����
| 2 |
| 3 |
�Ӧ��CO��CO2�����ʵ����ֱ�Ϊamol��bmol�����ݶ���֮������ԭ���غ��з��̣���a+b=2��a+2b=3��2-
| 45 |
| 18 |
�ʴ�Ϊ��3��1��3��
��3������ˮ�����ʵ���=
| 45g |
| 18g/mol |
| 2.5mol��2 |
| 4 |
| 5 |
| 4 |
| 2.5mol��2 |
| 6 |
| 5 |
| 6 |
| 5 |
| 6 |
| 5 |
| 4 |
�ʴ�Ϊ��
| 5 |
| 6 |
| 5 |
| 4 |
���������⿼���л���Ľṹ�����ʺͻ������йؼ��㣬Ϊ��Ƶ���㣬������ϩ���ʼ��ӳɷ�Ӧ�Ŀ��飬ע��������������������;�Ĺ�ϵ��ע������ԭ���غ��뼫�����еĽ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д�
�����Ŀ
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ���ǣ�������
A��Cu�����ϡ�����ڼ���ʱ�ķ�Ӧ��3Cu+2NO3-+8H+
| ||||
| B������������ˮ������ˮ��Cl2+H2O?2H++Cl-+ClO- | ||||
| C������FeCl3��Һ��Ӧ��2Fe+Fe3+�T3Fe2+ | ||||
| D����Ca��ClO��2��Һ��ͨ������CO2��Ca2++CO2+H2O�TCaCO3��+2H+ |
�������и�����Һ�У����ܴ���������������ǣ�������
| A��pH=0����Һ�У�Fe2+��NO3-��SO42-��I- |
| B����ˮ�������c��H+��=1��10-13mol?L-1����Һ�У�Na+��AlO2-��S2-��CO32- |
| C�����д���Fe3+����Һ�У�Na+��I-��K+��NO3- |
| D����ʹpH��ֽ�Ժ�ɫ����Һ��Mg2+��SO42-��Na+��Cl- |
 ������ʾA��B��C��D��E�仯��ϵ������ʡȥ�˷�Ӧ����������
������ʾA��B��C��D��E�仯��ϵ������ʡȥ�˷�Ӧ���������� 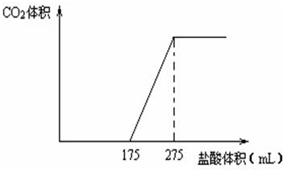 ȡNaHCO3��Na2O2�Ĺ�������x g����һ�ܱ������м�����250�棬��ַ�Ӧ���ų��������壮����Ӧ��Ĺ���ֳ���ȫ��ͬ�����ݣ�������һ��Ͷ�뵽������BaCl2��Һ�У����ɵõ�3.94g��������һ������������ˮ��������ų�������ˮ��Һ�л�����μ���ij���ʵ���Ũ�ȵ����ᣬ���������������������֮��Ĺ�ϵ����ͼ��ʾ���Իش��������⣺
ȡNaHCO3��Na2O2�Ĺ�������x g����һ�ܱ������м�����250�棬��ַ�Ӧ���ų��������壮����Ӧ��Ĺ���ֳ���ȫ��ͬ�����ݣ�������һ��Ͷ�뵽������BaCl2��Һ�У����ɵõ�3.94g��������һ������������ˮ��������ų�������ˮ��Һ�л�����μ���ij���ʵ���Ũ�ȵ����ᣬ���������������������֮��Ĺ�ϵ����ͼ��ʾ���Իش��������⣺ ��1����֪����298K��1.01KPa�£����ȶ��ĵ��ʷ�����Ӧ����1mol��̬������ķ�Ӧ�Ƚиû�����������ȣ���H������ͼ��ʾΪ����Ԫ���⻯��a��b��c��d������������ʾ��ͼ���Իش��������⣺������ɳ��ǽ���Ԫ���⻯����ȶ������䷴Ӧ�ȡ�H�Ĺ�ϵ
��1����֪����298K��1.01KPa�£����ȶ��ĵ��ʷ�����Ӧ����1mol��̬������ķ�Ӧ�Ƚиû�����������ȣ���H������ͼ��ʾΪ����Ԫ���⻯��a��b��c��d������������ʾ��ͼ���Իش��������⣺������ɳ��ǽ���Ԫ���⻯����ȶ������䷴Ӧ�ȡ�H�Ĺ�ϵ