��Ŀ����
16�� ��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺
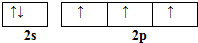
��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺��1����̬��ԭ�Ӻ��������5�ֲ�ͬ���˶�״̬����̬��ԭ�ӵļ۲�����Ų�ͼΪ
 ��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3��
��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3����2��������H3BNH3��һ��DZ�ڵĴ�����ϣ������û�����B3N3H6ͨ�����·�Ӧ�Ƶã�3CH4+2B3N3H6+6H2O�T3CO2+6H3BNH3
��H3BNH3�������Ƿ������λ���ǣ���ǡ�����B��C��N��O�ĵ�һ��������С�����˳��ΪN��O��C��B��
����B3N3H6��Ϊ�ȵ�����ķ�����C6H6����һ�����ɣ���B3N3H6Ϊ�Ǽ��Է��ӣ����ݵȵ���ԭ��д��B3N3H6�Ľṹʽ
 ��
����3�����϶���š�̽��������̫���ܵ�ذ��ṩ��������̫���ܵ�ذ�����г��������⣬����ͭ�������أ����Ȼ�ѧ���ʣ��ش��������⣺
��SeO3���ӵ����幹��Ϊƽ�������Σ�
�ڽ���ͭͶ�백ˮ��H2O2��Һ�о�����������Ͷ�백ˮ��H2O2�Ļ����Һ�У���ͭƬ�ܽ⣬��Һ������ɫ��д���÷�Ӧ�����ӷ�Ӧ����ʽΪCu+H2O2+4NH3•H2O=Cu��NH3��42++2OH-+4H2O��
��ij��ͭ�Ͻ�ľ����ṹ��ͼ��ʾ���þ����о��������ͭԭ�Ӻ͵�ԭ�Ӽ�ľ���Ϊ$\frac{\sqrt{2}}{2}$a pm����þ�����ܶ�Ϊ$\frac{206}{{N}_{A}��2\sqrt{2��{a}^{3}}}��1{0}^{30}$g•cm-3���ú�a�Ĵ���ʽ��ʾ����NAΪ����٤��������ֵ����
���� ��1����Ϊ5��Ԫ�أ���ԭ�ӵļ۲������5����CH3��2NNH2��Nԭ�ӵļ۲���Ӷ���Ϊ$\frac{5+3}{2}$=4���ݴ��ж�N���ӻ���ʽ��
��2���ٸ���B���������������γɵĹ��ۼ����жϣ�ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����IIA�塢��VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ�
��ԭ������ͬ������������ͬ�ķ��ӣ�����Ϊ�ȵ����壻���ݵȵ�����ṹ����ԭ��ȷ��B3N3H6�Ľṹʽ��
��3������̬SeO3����������ԭ�ӵļ۲���Ӷ��������жϷ��ӹ��ͣ�
�ڸ���������ԭ��Ӧ��Ԫ�غ͵���غ㣬��д�����ӷ���ʽ��
�۾����о��������ͭԭ�Ӻ͵�ԭ�Ӽ�ľ���Ϊ$\frac{\sqrt{2}}{2}$apm�����Ծ����ı߳�Ϊ$\sqrt{2}$apm���������Ϊ��$\sqrt{2}$apm��3�����þ�̯�����㾧���к��е�ͭԭ�Ӻ͵�ԭ�Ӹ��������ݦ�=$\frac{m}{V}$���㣮
��� �⣺��1����Ϊ5��Ԫ�أ���ԭ�ӵļ۲������5�����Ի�̬��ԭ�Ӻ�������� 5�ֲ�ͬ���˶�״̬����̬��ԭ�ӵļ۲�����Ų�ͼΪ ����CH3��2NNH2��Nԭ�ӵļ۲���Ӷ���Ϊ$\frac{5+3}{2}$=4������N���ӻ���ʽΪsp3�ӻ���
����CH3��2NNH2��Nԭ�ӵļ۲���Ӷ���Ϊ$\frac{5+3}{2}$=4������N���ӻ���ʽΪsp3�ӻ���
�ʴ�Ϊ��5�� ��sp3��
��sp3��
��2����B������������Ϊ3�����γɵ�3�����ۼ���������A��H3BNH3����B��H�γ�3�����ۼ���Bԭ�ӵĿչ����Nԭ�ӵŶԵ����γ���λ����ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����IIA�塢��VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ��⼸��Ԫ�ض��ǵڶ�����Ԫ�أ�B��C��N��O���������ֱ��ǣ���IIIA�塢��IVA�塢��VA�塢��VIA�壬�������ǵĵ�һ�����ܴ�С˳����N��O��C��B��
�ʴ�Ϊ���ǣ�N��O��C��B��
��ԭ������ͬ������������ͬ�ķ��ӣ�����Ϊ�ȵ����壬�루HB=NH��3��Ϊ�ȵ�����ķ���ΪC6H6��B3N3H6�Ľṹʽ�뱽���ƣ���ṹʽΪ ��
��
�ʴ�Ϊ��C6H6�� ��
��
��3������̬SeO3����������ԭ�ӵļ۲���Ӷ���Ϊ$\frac{6+0}{2}$=3���µ��Ӷԣ����Է��ӹ���Ϊƽ�������Σ�
�ʴ�Ϊ��ƽ�������Σ�
�ڽ���Cu�����백ˮ����������ⶼ���ܷ�Ӧ�������백ˮ��������Ļ����Һ��Ӧ��˵�������ܻ���ٽ������������ʹ�ͬ���õĽ�������й�������Ϊ������������Cu2+�γ������ӣ�������ٽ�ʹ��Ӧ���У�����ʽ�ɱ�ʾΪ��Cu+H2O2+4NH3•H2O=Cu��NH3��42++2OH-+4H2O��
�ʴ�Ϊ��Cu+H2O2+4NH3•H2O=Cu��NH3��42++2OH-+4H2O��
���ھ����У�Nԭ��λ�ڶ��㣬Cuԭ��λ������е㣬�þ�����Nԭ�Ӹ���=8��$\frac{1}{8}$=1��Cuԭ�Ӹ���=12��$\frac{1}{4}$=3���������V=��a��10-10cm��3�������ı߳�Ϊ$\sqrt{2}$apm���������Ϊ��$\sqrt{2}$apm��3�����=$\frac{\frac{64��3+14}{{N}_{A}}}{��{\sqrt{2}a��1{0}^{-10}��}^{3}}$g•cm-3=$\frac{206}{{N}_{A}��2\sqrt{2��{a}^{3}}}��1{0}^{30}$g•cm-3��
�ʴ�Ϊ��$\frac{206}{{N}_{A}��2\sqrt{2��{a}^{3}}}��1{0}^{30}$g•cm-3��
���� ���⿼�������ʽṹ�������ʣ��漰ԭ���ӻ���ʽ���жϡ��۵����Ų�ʽ����д�������ļ����֪ʶ�㣬��Ŀ�ۺ��Խ�ǿ��ע����ݼ۲���ӶԻ������ۡ�����ԭ����֪ʶ�����������Ŀ�Ѷ��еȣ�

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�| A�� | Ԫ��Y��Z��W������ͬ���Ӳ�ṹ�����ӣ���뾶�������� | |
| B�� | Ԫ��Y����Ԫ��Z�γɻ�����Z2Y2���û������ڲ������ֻ�ѧ�� | |
| C�� | Ԫ��Y��R�ֱ���Ԫ��X�γɵĻ���������ȶ��ԣ�XmY��XmR���е�XmY��XmR | |
| D�� | Ԫ��W��R������������ˮ���ﶼ��ǿ�� |
| A�� | ��Ʒ���б��������H2SO4 | B�� | �в���δ��Ӧ���Ҵ���������� | ||
| C�� | �в���δ��Ӧ�����ᱻ������� | D�� | �в����Ҵ���Ũ������������CO2 |

| A�� | �����;���ڣ�;���ٸ��õ���������ɫ��ѧ˼�� | |
| B�� | X������SO2��SO3�Ļ������ | |
| C�� | Y��������������Һ | |
| D�� | ��CuSO4��Һ�����������������ɣ����Ƶõ������� |
| A�� | c��Na+��=c��S2-�� | B�� | c��Na+��=2c��S2-�� | C�� | c��Na+����c��S2-����2��1 | D�� | c��Na+����c��S2-����2��1 |
| A�� | Al3+ Na+ NO3- Cl- | B�� | K+ Na+ Cl- NO3- | ||
| C�� | K+ Na+ Cl- AlO2- | D�� | K+ NH4+ SO42- NO3- |


 ����дһ�֣����������������ۻ����ϩ�Ĺ�ϵ��B��������ĸ��
����дһ�֣����������������ۻ����ϩ�Ĺ�ϵ��B��������ĸ�� ����
���� ����д�����з�Ӧ���л�����Ľṹ��ʽ��
����д�����з�Ӧ���л�����Ľṹ��ʽ�� OHC-CHO��OHC-CH2-CHO��
OHC-CHO��OHC-CH2-CHO��