��Ŀ����
0.3mol����̬����ȼ�������飨B2H6����������ȼ�գ����ɹ�̬�����������ˮ�������ų�609.9kJ����������֪��H2O (l ) == H2O��g�� ��H =" +44" kJ��mol-1�������ܱ�ʾ������ȼ���ȵ��Ȼ�ѧ����ʽ���ǣ� ��
| A��1/3B2H6 (g) + O2 (g) ==1/3B2O3 (g) + H2O (g)��H =" -677.7" kJ��mol-1 |
| B��B2H6 (g) + 3O2 (g)="=" B2O3(s) + 3H2O (g)��H =" -2165" kJ��mol-1 |
| C��B2H6 (g) + 3O2 (g)="=" B2O3(s) + 3H2O (g)��H =" -2033" kJ��mol-1 |
| D��B2H6 (g) + 3O2 (g)== B2O3(s) + 3H2O��l����H =" -2165" kJ��mol-1 |
D
��һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų���������ȼ���ȣ�����ˮ���ȶ�״̬��Һ̬������ֻ��ѡ��D����ȷ�ģ�����Ǵ���ģ���ѡD��

��ϰ��ϵ�д�
�����Ŀ


 (aq)��Ba2��(aq)��2OH��(aq)===BaSO4(s)��2H2O(l)����H����114.6 kJ��mol��1
(aq)��Ba2��(aq)��2OH��(aq)===BaSO4(s)��2H2O(l)����H����114.6 kJ��mol��1 2NH3(g)��H="��38.6" kJ��mol��1
2NH3(g)��H="��38.6" kJ��mol��1 ��2H2O��g�� ��H2����483.6 kJ��
��2H2O��g�� ��H2����483.6 kJ��
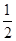 O2��g��=== ZnO(s)����H=" -348.3" kJ��mol-1��
O2��g��=== ZnO(s)����H=" -348.3" kJ��mol-1��