��Ŀ����
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�ء���֪H2(g)��CO(g)��CH3OH(l)��ȼ���ȡ�H�ֱ�Ϊ-285.8kJ��mol-1��-283.0kJ��mol-1��-726.5kJ��mol-1����ش��������⣺
��1����̫���ֽܷ�10molҺ̬ˮ���ĵ�������__________kJ��
��2���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ________________________________��
��3�����ݻ�Ϊ2L���ܱ������У���һ������CO2��H2�ϳɼ״����������������������£��¶ȶԷ�Ӧ��Ӱ�죬ʵ��������ͼ��ʾ��ע��T1��T2������300�棩������˵����ȷ����___________������ţ�

�� �¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״���ƽ������Ϊv(CH3OH)=  mol��L-1��min-1
mol��L-1��min-1
�� �÷�Ӧ��T1ʱ��ƽ�ⳣ����T2ʱ�Ĵ�
�� �÷�Ӧ�� ��H < 0
�� ����A��ķ�Ӧ��ϵ��T1�䵽T2���ﵽƽ��ʱ�����������ܶȼ�С
�� ����A��ʱ�����е�ѹǿ�ȴ���B��ʱ�����е�ѹǿ��
��4����T1�¶�ʱ����1molCO2��3molH2����һ�ܱպ������У���ַ�Ӧ�ﵽƽ�����CO2ת����Ϊa, �������ڵ�ѹǿ����ʼѹǿ֮��Ϊ___________��
��5����ֱ���Լ״�Ϊȼ�ϵĵ���У��������ҺΪ���ԣ������ķ�ӦʽΪ________________________��
��1��________2858______________kJ��
��2��____________ CH3OH(l)+O2(g)=CO(g)+2H2O(l) ��H =����443.5kJ��mol-1 ____��
��3��_________�ڢ�__________������ţ�
��4�� _____1��������/2 ��_____��
��5�� CH3OH��6e-+��H2O=��CO2+6H�� ___
����������

 ��2011?��������ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1����ش��������⣺
��2011?��������ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1����ش��������⣺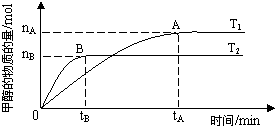 ��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1�� ̼�ǻ�������������Ԫ�أ��䵥�ʼ������������������������Ҫ��Դ���ʣ�
̼�ǻ�������������Ԫ�أ��䵥�ʼ������������������������Ҫ��Դ���ʣ�