��Ŀ����
 ��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1����ش��������⣺
��1����̫���ֽܷ�5molˮ���ĵ�������
1429
1429
kJ����2���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ
CH3OH��l��+O2��g��=CO��g��+2 H2O��l����H=-443.5kJ?mol-1
CH3OH��l��+O2��g��=CO��g��+2 H2O��l����H=-443.5kJ?mol-1
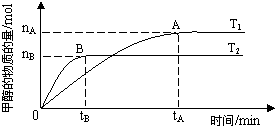
����3�����ݻ�Ϊ2L���ܱ������У���CO2��H2�ϳɼ״����������������������£������¶ȶԷ�Ӧ��Ӱ�죬ʵ������ͼ��ʾ��ע��T1��T2������300�棩��
����˵����ȷ����
�ڢ�
�ڢ�
������ţ��ٶ�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״���ƽ������Ϊ��v��CH3OH��=
| nA |
| tA |
�ڸ÷�ӦΪ���ȷ�Ӧ
�۸÷�Ӧ��T1ʱ��ƽ�ⳣ����T2ʱ��С
�ܴ���A��ķ�Ӧ��ϵ��T1�䵽T2���ﵽƽ��ʱ
| n(H2) |
| n(CH3OH) |
��4����T1�¶�ʱ����1molCO2��3molH2����һ�ܱպ��������У���ַ�Ӧ�ﵽƽ�����CO2ת����Ϊa���������ڵ�ѹǿ����ʼѹǿ֮��Ϊ
��2-a����2
��2-a����2
����������1������������ȼ���ȿ�֪ˮ�ֽ����յ�������Ȼ�����û�ѧ�������뷴Ӧ�ȵĹ�ϵ�����㣻
��2������CO��CH3OH��ȼ��������д�ȷ���ʽ�������ø�˹�����������״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��
��3������ͼ���м״��ı仯�����㷴Ӧ���ʣ�������ͼ����ʱ�������ʵĹ�ϵ������T1��T2��������Ӱ��ƽ����������������
��4�����ݻ�ѧƽ������η�����ƽ��ʱ�����ʵ����ʵ����������÷�Ӧǰ����������ʵ���֮�ȵ���ѹǿ֮�������
��2������CO��CH3OH��ȼ��������д�ȷ���ʽ�������ø�˹�����������״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��
��3������ͼ���м״��ı仯�����㷴Ӧ���ʣ�������ͼ����ʱ�������ʵĹ�ϵ������T1��T2��������Ӱ��ƽ����������������
��4�����ݻ�ѧƽ������η�����ƽ��ʱ�����ʵ����ʵ����������÷�Ӧǰ����������ʵ���֮�ȵ���ѹǿ֮�������
����⣺��1����H2��g����ȼ���ȡ�HΪ-285.8kJ?mol-1֪��1molH2��g����ȫȼ������1molH2O��l���ų�����285.8kJ��
���ֽ�1mol H2O��l��Ϊ1mol H2��g�����ĵ�����Ϊ285.8kJ����ֽ�5mol H2O��l�����ĵ�����Ϊ285.8kJ��5=1429kJ��
�ʴ�Ϊ��1429��
��2����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-283.0kJ?mol-1��-726.5kJ?mol-1����
��CO��g��+
O2��g��=CO2��g����H=-283.0kJ?mol-1
��CH3OH��l��+
O2��g��=CO2��g��+2 H2O��l����H=-726.5kJ?mol-1
�ɸ�˹���ɿ�֪����-�ٵ÷�ӦCH3OH��l��+O2��g��=CO��g��+2 H2O��l�������H=-726.5kJ?mol-1-��-283.0kJ?mol-1��=-443.5kJ?mol-1��
�ʴ�Ϊ��CH3OH��l��+O2��g��=CO��g��+2 H2O��l����H=-443.5kJ?mol-1��
��3���������ͼ�������֪��T2�ȴﵽƽ����T2��T1�����¶����߷�Ӧ���������֪T2�ķ�Ӧ���ʴ���T1�����¶ȸ�ʱƽ��״̬CH3OH�����ʵ����٣���˵�����淴ӦCO2+3H2?CH3OH+H2O���淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ����T1ʱ��ƽ�ⳣ����T2ʱ�Ĵ�
��v��CH3OH��=
=
mol/L?min���ʢٴ���
������ͼ�����T2�ȴﵽƽ����T2��T1�����¶����߷�Ӧ���������֪T2�ķ�Ӧ���ʴ���T1�����¶ȸ�ʱƽ��״̬CH3OH�����ʵ����٣���˵�����淴ӦCO2+3H2?CH3OH+H2O���淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ���ʢ���ȷ��
������ӦΪ���ȷ�Ӧ���������ͼ�������֪��T2�ȴﵽƽ����T2��T1�������¶ȣ�ƽ�����淴Ӧ�����ƶ����÷�Ӧ��T1ʱ��ƽ�ⳣ����T2ʱ�Ĵʢ۴���
�ܴ���A��ķ�Ӧ��ϵ��T1�䵽T2�������¶ȣ�ƽ�����淴Ӧ�����ƶ����ﵽƽ��ʱ���������ʵ������״������ʵ�����С����
���ʢ���ȷ��
�ʴ�Ϊ���ڢܣ�
��4���ɻ�ѧƽ�������ģʽ�������֪��
CO2 ��g��+3H2��g��=CH3OH��g��+H2O��g��
��ʼ 1 3 0 0
�仯 a 3a a a
ƽ�� 1-a 3-3a a a
������ͬ�����������ѹǿ֮�ȵ������ʵ���֮�ȣ�
�������ڵ�ѹǿ����ʼѹǿ֮��Ϊ=��1-a+3-3a+a+a������1+3��=��2-a����2��
�ʴ�Ϊ����2-a����2��
���ֽ�1mol H2O��l��Ϊ1mol H2��g�����ĵ�����Ϊ285.8kJ����ֽ�5mol H2O��l�����ĵ�����Ϊ285.8kJ��5=1429kJ��
�ʴ�Ϊ��1429��
��2����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-283.0kJ?mol-1��-726.5kJ?mol-1����
��CO��g��+
| 1 |
| 2 |
��CH3OH��l��+
| 1 |
| 2 |
�ɸ�˹���ɿ�֪����-�ٵ÷�ӦCH3OH��l��+O2��g��=CO��g��+2 H2O��l�������H=-726.5kJ?mol-1-��-283.0kJ?mol-1��=-443.5kJ?mol-1��
�ʴ�Ϊ��CH3OH��l��+O2��g��=CO��g��+2 H2O��l����H=-443.5kJ?mol-1��
��3���������ͼ�������֪��T2�ȴﵽƽ����T2��T1�����¶����߷�Ӧ���������֪T2�ķ�Ӧ���ʴ���T1�����¶ȸ�ʱƽ��״̬CH3OH�����ʵ����٣���˵�����淴ӦCO2+3H2?CH3OH+H2O���淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ����T1ʱ��ƽ�ⳣ����T2ʱ�Ĵ�
��v��CH3OH��=
| ||
| tA |
| nA |
| 2tA |
������ͼ�����T2�ȴﵽƽ����T2��T1�����¶����߷�Ӧ���������֪T2�ķ�Ӧ���ʴ���T1�����¶ȸ�ʱƽ��״̬CH3OH�����ʵ����٣���˵�����淴ӦCO2+3H2?CH3OH+H2O���淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ���ʢ���ȷ��
������ӦΪ���ȷ�Ӧ���������ͼ�������֪��T2�ȴﵽƽ����T2��T1�������¶ȣ�ƽ�����淴Ӧ�����ƶ����÷�Ӧ��T1ʱ��ƽ�ⳣ����T2ʱ�Ĵʢ۴���
�ܴ���A��ķ�Ӧ��ϵ��T1�䵽T2�������¶ȣ�ƽ�����淴Ӧ�����ƶ����ﵽƽ��ʱ���������ʵ������״������ʵ�����С����
| n(H2) |
| n(CH3OH) |
�ʴ�Ϊ���ڢܣ�
��4���ɻ�ѧƽ�������ģʽ�������֪��
CO2 ��g��+3H2��g��=CH3OH��g��+H2O��g��
��ʼ 1 3 0 0
�仯 a 3a a a
ƽ�� 1-a 3-3a a a
������ͬ�����������ѹǿ֮�ȵ������ʵ���֮�ȣ�
�������ڵ�ѹǿ����ʼѹǿ֮��Ϊ=��1-a+3-3a+a+a������1+3��=��2-a����2��
�ʴ�Ϊ����2-a����2��
������������Ҫ������ȼ���ȼ��㡢��˹���ɡ��Ȼ�ѧ��Ӧ����ʽ����Ӧ���ʡ���ѧƽ�����Ҫ֪ʶ���Ѷ��еȣ�ע��Ի���֪ʶ���������գ�

��ϰ��ϵ�д�
�����Ŀ
 ��2011?��������ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1����ش��������⣺
��2011?��������ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1����ش��������⣺ ̼�ǻ�������������Ԫ�أ��䵥�ʼ������������������������Ҫ��Դ���ʣ�
̼�ǻ�������������Ԫ�أ��䵥�ʼ������������������������Ҫ��Դ���ʣ�