��Ŀ����
ʵ������������ƿ�м�NaBr������ˮ��95�����Ҵ���ŨH2SO4���߷�Ӧ��������������������ˮ���ռ�����ã���ӦʽΪ��NaBr+H2SO4=NaHSO4+HBr
C2H5OH+HBr=C2H5Br+H2O
���ܷ����ĸ���Ӧ�У�2HBr+H2SO4(Ũ)=Br2+SO2��+2H2O����֪C2H5Br�ķе�Ϊ38.4�棬�ܶȱ�ˮ��Ϊ�����²�����ˮ����״Һ�壮
��ش��������⣺
(1)��Ӧ�м�������ˮ����Ϊ���ܽ�NaBr�⣬���û��Т�____________����____________��
(2)Ϊ�˱�֤�����������ȣ����ȷ�����ò���________________________��
(3)��ƿ�еĵ�������ƿ���ϵĴ�ֱ���ֱ�����ͬ��װ���е���Ҫ����ԭ��__________
_________��
(4)��ʵ����ñ߷�Ӧ������IJ�����ԭ����_________________��
(5)���������ˮ���ռ�����õ����ݺʹ�ˮ�з�����ķ�����___________________��
�𰸣�
������
������
| (1)��ϡ��ŨH2SO4��ֹŨH2SO4����HBr������Br2,���ܽ�HBr
(2)ˮԡ���� (3)���������������� (4)�����CH3CH2Br�������������Ũ�ȣ�ʹƽ�������ƶ� (5)���ݢ�CH3CH2Br������ˮ����ˮ�� �������ջӷ����Ҵ���������Һ
|

��ϰ��ϵ�д�
�����Ŀ
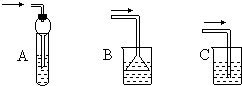 ʵ������������ƿ�м�NaBr������ˮ��95%���Ҵ���Ũ���ᣬ�߷�Ӧ��������������������ˮ���ռ�����ã���Ӧ�Ļ�ѧ����ʽΪ��
ʵ������������ƿ�м�NaBr������ˮ��95%���Ҵ���Ũ���ᣬ�߷�Ӧ��������������������ˮ���ռ�����ã���Ӧ�Ļ�ѧ����ʽΪ��

 C2H5Br+H2O
C2H5Br+H2O