��Ŀ����
ʵ������������ƿ�м�NaBr������ˮ��95�����Ҵ���ŨH2SO4���߷�Ӧ��������������������ˮ���ռ�����á���ӦʽΪ��![]()
![]()
���п��ܷ����ĸ���Ӧ�У�2HBr��H2SO4��Ũ��=====Br2��SO2����2H2O����֪CH3CH2Br�ķе�Ϊ38��4�棬�ܶȱ�ˮ��Ϊ�����²�����ˮ����״Һ�塣
��ش��������⣺
��1����Ӧ�м���������ˮ����Ϊ���ܽ�NaBr�⣬���û��У���________����________��
��2��Ϊ�˱�֤�����������ȺͿ��ƺ��£����ȷ�����ò���________��
��3����ƿ�еĵ�������ƿ���ϵĴ�ֱ���ֱ�����ͬ��װ���еĵ���Ҫ��Щ��ԭ����________��
��4����ʵ���Ƿ���Ҫ���ñ߷�Ӧ������IJ������?
��5�����������ˮ���ռ�����õ����ݺʹ�ˮ�з���ķ�����________��
��6������װ����ʵ���м����������壬���ܷ�ֹҺ�嵹������________�����ţ���

������
��1��������HBr,��ϡ��ŨH2SO4�������������ԣ����ٸ����� ��2��ˮԡ���� ��3��ʹ�е�����������������ȴ���������ٷ�Ӧ�����ʧ�����������IJ����ʹ��� ��4����Ҫ�� ��5�������鲻����ˮ�ұ�ˮ�أ����÷�Һ©����Һ ��6��B��C��D��E
|

 �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�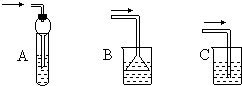 ʵ������������ƿ�м�NaBr������ˮ��95%���Ҵ���Ũ���ᣬ�߷�Ӧ��������������������ˮ���ռ�����ã���Ӧ�Ļ�ѧ����ʽΪ��
ʵ������������ƿ�м�NaBr������ˮ��95%���Ҵ���Ũ���ᣬ�߷�Ӧ��������������������ˮ���ռ�����ã���Ӧ�Ļ�ѧ����ʽΪ��
 C2H5Br+H2O
C2H5Br+H2O