��Ŀ����
��֪�����ǻ�ͬʱ����ͬһ��̼ԭ����ʱ�ṹ�Dz��ȶ��ģ�����������ˮ��Ӧ�����磺CH3CH��OH��2��CH3CHO+H2O�����з���ʽΪC9H8O2Br2������M����֪E�Ļ�ѧʽΪC7H5O2Na��A����Է�������Ϊ46����һ�������¿ɷ�������һϵ�з�Ӧ��

��ش���������
��1��B�й����ŵ����� ��A�ĺ˴Ź��������� �����շ壻G��H�ķ�Ӧ���� ��
��2��M�Ľṹ��ʽ
��3��д�����з�Ӧ�Ļ�ѧ����ʽ
��E��F ��H��I
��4��ͬʱ��������������G��ͬ���칹��Ľṹ��ʽ�� �֣�д������һ��
A�������к��б��� B �ܷ���ˮ�ⷴӦC���ܷ���������Ӧ D��FeCl3��Һ��Ӧ����ɫ��

��ش���������
��1��B�й����ŵ�����
��2��M�Ľṹ��ʽ
��3��д�����з�Ӧ�Ļ�ѧ����ʽ
��E��F
��4��ͬʱ��������������G��ͬ���칹��Ľṹ��ʽ��
A�������к��б��� B �ܷ���ˮ�ⷴӦC���ܷ���������Ӧ D��FeCl3��Һ��Ӧ����ɫ��
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
������A����Է�������Ϊ46��A��������B��B�ɼ���������C����B��������C������AΪCH3CH2OH��BΪCH3CHO��CΪCH3COOH��E�Ļ�ѧʽΪC7H5O2Na������������ͭ��Һ��Ӧ����ש��ɫ������˵��E�к���-CHO��Hת��ΪIʱ������ֻ��һ�ֽṹ��˵��D��E��F��H��I�б����ϵ�ȡ�������ڶ�λ����EΪ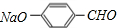 �����������ǿ�ڷӣ��ڼ��������£������л����ת����֪FΪ
�����������ǿ�ڷӣ��ڼ��������£������л����ת����֪FΪ ��GΪ
��GΪ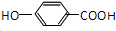 ��HΪ
��HΪ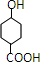 ����IΪ
����IΪ ��DӦΪBr2CH-
��DӦΪBr2CH- -OH��MΪ��
-OH��MΪ�� ������л���Ľṹ�����ʿɽ����⣮
������л���Ľṹ�����ʿɽ����⣮
 �����������ǿ�ڷӣ��ڼ��������£������л����ת����֪FΪ
�����������ǿ�ڷӣ��ڼ��������£������л����ת����֪FΪ ��GΪ
��GΪ ��HΪ
��HΪ ����IΪ
����IΪ ��DӦΪBr2CH-
��DӦΪBr2CH- -OH��MΪ��
-OH��MΪ�� ������л���Ľṹ�����ʿɽ����⣮
������л���Ľṹ�����ʿɽ����⣮���
�⣺A����Է�������Ϊ46��A��������B��B�ɼ���������C����B��������C������AΪCH3CH2OH��BΪCH3CHO��CΪCH3COOH��E�Ļ�ѧʽΪC7H5O2Na������������ͭ��Һ��Ӧ����ש��ɫ������˵��E�к���-CHO��Hת��ΪIʱ������ֻ��һ�ֽṹ��˵��D��E��F��H��I�б����ϵ�ȡ�������ڶ�λ����EΪ �������л����ת����֪FΪ
�������л����ת����֪FΪ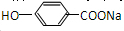 ��GΪ
��GΪ ��HΪ
��HΪ ����IΪ
����IΪ ��DӦΪBr2CH-
��DӦΪBr2CH- -OH��MΪ��
-OH��M�� ��
��
��1��BΪCH3CHO������ȩ����AΪCH3CH2OH������3�����ʲ�ͬ��H���˴Ź���������3�����շ壬G��H��GΪ ��ͨ���ӳɷ�Ӧ������
��ͨ���ӳɷ�Ӧ������ ��
��
�ʴ�Ϊ��ȩ���� 3���ӳɷ�Ӧ��
��2�������Ϸ�����֪MΪ ��
��
�ʴ�Ϊ�� ��
��
��3����EΪ ���ڼ�����������������ͭ��Ӧ����FΪ
���ڼ�����������������ͭ��Ӧ����FΪ ����Ӧ�ķ���ʽ
����Ӧ�ķ���ʽ ��
��
�ʴ�Ϊ�� ��
��
��HΪ ��ͨ����ȥ��Ӧ������
��ͨ����ȥ��Ӧ������ ����Ӧ�ķ���ʽΪ
����Ӧ�ķ���ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��4��GΪ ����������֪G��ͬ���칹��Ӧ����-COO-��-CHO���Լ����ǻ������������µ���
����������֪G��ͬ���칹��Ӧ����-COO-��-CHO���Լ����ǻ������������µ���
 ���١��䡢����дһ�֣��Ƚṹ��
���١��䡢����дһ�֣��Ƚṹ��
�ʴ�Ϊ��3�� ��
��
 �������л����ת����֪FΪ
�������л����ת����֪FΪ ��GΪ
��GΪ ��HΪ
��HΪ ����IΪ
����IΪ ��DӦΪBr2CH-
��DӦΪBr2CH- -OH��MΪ��
-OH��M�� ��
����1��BΪCH3CHO������ȩ����AΪCH3CH2OH������3�����ʲ�ͬ��H���˴Ź���������3�����շ壬G��H��GΪ
 ��ͨ���ӳɷ�Ӧ������
��ͨ���ӳɷ�Ӧ������ ��
���ʴ�Ϊ��ȩ���� 3���ӳɷ�Ӧ��
��2�������Ϸ�����֪MΪ
 ��
���ʴ�Ϊ��
 ��
����3����EΪ
 ���ڼ�����������������ͭ��Ӧ����FΪ
���ڼ�����������������ͭ��Ӧ����FΪ ����Ӧ�ķ���ʽ
����Ӧ�ķ���ʽ ��
���ʴ�Ϊ��
 ��
����HΪ
 ��ͨ����ȥ��Ӧ������
��ͨ����ȥ��Ӧ������ ����Ӧ�ķ���ʽΪ
����Ӧ�ķ���ʽΪ ��
���ʴ�Ϊ��
 ��
����4��GΪ
 ����������֪G��ͬ���칹��Ӧ����-COO-��-CHO���Լ����ǻ������������µ���
����������֪G��ͬ���칹��Ӧ����-COO-��-CHO���Լ����ǻ������������µ��� ���١��䡢����дһ�֣��Ƚṹ��
���١��䡢����дһ�֣��Ƚṹ���ʴ�Ϊ��3��
 ��
��
���������⿼���л�����ƶϣ��漰����ȩ�����ᡢ±�����������ӵ����ʵȣ���Ŀ�ѶȽϴ���Ҫ�Ը������Ϣ�������ã��ܽϺõĿ��鿼�����Ķ�����ѧ������˼ά���������ȵ����ͣ���ѧ�����������нϸߵ�Ҫ�����չ����ŵ������ǹؼ���ע����������Ϣ����ȡ���Ʒ������Ʒ����ϵķ����ƶϣ�

��ϰ��ϵ�д�
�����Ŀ
�����£����и���������ָ����Һ���ܴ���������ǣ�������
| A����ˮ���������c��H+��=10-13mol/L����ɫ��Һ�У�ClO-��Na+��HCO3-��Mg2+ |
| B��ʹpH��ֽ��Ϊ����ɫ����Һ�У�K+��CO32-��Na+��AlO2- |
| C������Mg�ܷų�H2����Һ�У�Mg2+��NH4+��NO3-��Cl- |
| D��ʹ���ȱ�����Һ�У�MnO4-��NO3-��Na+��Fe2+ |
 2008���ļ����˻ἴ�����ҹ����У��ҹ�֧�֡���ɫ���ˡ��Ƽ����ˡ����İ��ˡ������İ��˵�һ����Ҫ�����Ǽ�������˶�Ա�����˷ܼ���ij���˷ܼ��Ľṹ��ʽ��ͼ���йظ����ʵ�˵����ȷ���ǣ�������
2008���ļ����˻ἴ�����ҹ����У��ҹ�֧�֡���ɫ���ˡ��Ƽ����ˡ����İ��ˡ������İ��˵�һ����Ҫ�����Ǽ�������˶�Ա�����˷ܼ���ij���˷ܼ��Ľṹ��ʽ��ͼ���йظ����ʵ�˵����ȷ���ǣ�������| A�����ڷ����� |
| B�����ڸ߷��ӻ����� |
| C�����ڱ��ӵ�ͬϵ�� |
| D������ˮ�ɷ����ӳɷ�Ӧ��ȡ����Ӧ |
ij��Ԫ��H2A��ˮ�з������룺H2A=H++HA-����ȫ���룩��HA-?H++A2-������������һ������ȷ���ǣ�������
| A����NaHA��Һ�У�c��Na+����c��HA-����c��OH-����c��H+�� |
| B����Na2A��Һ�У�c��Na+����c��A2-����c��OH-����c��H+�� |
| C����NaHA��Һ�У�c��H+��=c��A2-��+c��OH-�� |
| D����H2A��Һ�У�c��H+��=c��HA-��+c��OH-��+2c��A2-�� |


 ���ޣ�NH4��2CO3��
���ޣ�NH4��2CO3��