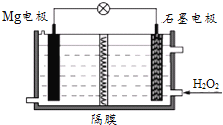
��Ŀ����
����Ŀ����1�����и������ʣ���C60��C70�����ʯ��ʯī�� �ڱ��״����Լ����ӡ��ڼ����ӡ�������ӣ���126C��136C��146C����HOCH2CHO��HOCH2CH2CHO��HOCH2CH2CH2CHO���������顢2,2���������飻 �״����Ҷ�����������������ͬλ�ص���_________������ͬϵ�����_________������ͬ���칹�����_________������ͬ�����������_________������ͬ�����ʵĵ���_________������������ţ�
��2������ͬϵ���У��еIJ����ܱ����Ը��������Һ���������ɷ����ᣬ��Ӧ���£�
 (R��R���ʾ�������ԭ��)��
(R��R���ʾ�������ԭ��)��
���б���ͬϵ��ס��ҡ���������ʽ����C10H14���ײ��ܱ����Ը��������Һ����Ϊ�����ᣬ���Ľṹ��ʽ��________�����ܱ����Ը��������Һ����Ϊ����ʽΪC8H6O4�ķ����ᣬ���ҿ��ܵĽṹ��________�֡����ı����ϵ�һ�����ֻ��һ�֡���д�����ֱ��Ľṹ��ʽ______________________________________________��
���𰸡��� �� �� �� ��  9
9 

��������
��1��ͬ��Ԫ�صIJ�ͬ���ػ�Ϊͬλ�أ��������ͬλ�ص��Ǣۣ�
�ṹ���ơ��������������ɸ���CH2��ԭ���ŵ��л��������Ϊͬϵ��������ͬϵ����Ǣܣ�
����ʽ��ͬ���ṹ��ͬ�Ļ����ﻥΪͬ���칹�壬�������ͬ���칹����Ǣڣ�
���Ԫ����ͬ���ṹ��ͬ�ĵ��ʻ�Ϊͬ�������壬�������ͬ����������Ǣ٣�
����ͬ�����ʵĵ��Ǣݣ�
��2���ܱ�����Ϊ������ı���ͬϵ�ﶼ��һ����ͬ��:�����뱽��������̼ԭ��������ԭ��,�������Ȼ���Ŀ���ڱ���ͬϵ��������ܱ������IJ�����Ŀ���ײ��ܱ����Ը��������Һ����Ϊ�����ᣬ���Ľṹ��ʽ�� �������ܱ�����Ϊ����ʽΪ
�������ܱ�����Ϊ����ʽΪ![]() �ķ�����(��Ԫ��),˵������������������,�����������һ�,Ҳ������һ������һ������,�������������ֽṹ
�ķ�����(��Ԫ��),˵������������������,�����������һ�,Ҳ������һ������һ������,�������������ֽṹ![]() ��
��![]() ,���������ڱ����ϵ�λ��������λ����λ�Ͷ�λ3�ֿ���,�ʷ���ʽΪ
,���������ڱ����ϵ�λ��������λ����λ�Ͷ�λ3�ֿ���,�ʷ���ʽΪ![]() ,�����������ı���ͬϵ����
,�����������ı���ͬϵ����![]() �ֽṹ������ʽΪ
�ֽṹ������ʽΪ![]() �ı���ͬϵ��ı����ϵ�һ�����ֻ��һ��,�䱽���Ͽ���ֻ��2����ͬ������λ�ڶ�λ,��4����ͬ�IJ��������ܵĽṹΪ��
�ı���ͬϵ��ı����ϵ�һ�����ֻ��һ��,�䱽���Ͽ���ֻ��2����ͬ������λ�ڶ�λ,��4����ͬ�IJ��������ܵĽṹΪ��
 .
.

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ij����С��������CuO��NH3��Ӧ���о�NH3��ij�����ʲ��ⶨ����ɣ����������ʵ��װ��(�г�װ��δ����)����ʵ�顣��ش��������⣺

(1)����a������Ϊ____������b�п�ѡ����Լ�Ϊ____��
(2)ʵ������,����װ��A��������ȡ����ɫ������____(����ĸ)��
A��Cl2 | B��O2 | C��CO2 | D��NO2 |
(3)ʵ���й۲쵽װ��C�к�ɫCuO��ĩ��Ϊ��ɫ���壬����������ɫ��ζ�������������������֤��NH3����____��,д����Ӧ�Ļ�ѧ����ʽ:_______________________��
(4)Eװ����Ũ�����������_____________________________________��
(5)��ȡ�������ǰ��Ӧ��װ��F���еIJ�����____________________________��
(6)ʵ����ϣ�����ø����D����m g��װ��F�����������Ϊn L��������ɱ�״�����������е������ԭ�Ӹ�����Ϊ____���ú�m��n��ĸ�Ĵ���ʽ��ʾ����