��Ŀ����
14������п�����������·ۣ�������ýȾ������������ľ�ķ������ȣ�����������п����Ҫ�ɷ�ΪZnO������ZnSiO3��FeCO3��CuO�ȣ�Ϊԭ������ZnSO4•7H2O������������ͼ��
��1������I�IJ�����������ˣ�����A����Ҫ�ɷ���H2SiO3��
��2��������У���pHԼΪ5.1����Һ�м��������أ����� Fe��OH��3��MnO��OH��2���ֳ������÷�Ӧ�����ӷ���ʽΪ3Fe2++MnO4-+8H2O=3Fe��OH��3��+MnO��OH��2��+5H+��
��3������IV�еĺ�ɲ������ڼ�ѹ���������½��У���ԭ���ǽ��ͺ�ɵ��¶ȣ���ֹZnSO4•7H2O�ֽ⣮
��4��ȡ28.70g ZnSO4•7H2O��������ͬ�¶ȣ�ʣ�����������仯��ͼ��ʾ��680��ʱ����Ļ�ѧʽΪ
b

a��ZnO b��Zn3O��SO4��2 c��ZnSO4 d��ZnSO4•H2O��
���� ����п����Ҫ�ɷ�ΪZnO������ZnSiO3��FeCO3��CuO�ȣ���ϡ�����ܽ⣬���ˣ���Һ�к�������п������ͭ�������������ټӸ��������Һ���������ӷ�Ӧ����MnO��OH��2�������������������ˣ���Һ�к�������ͭ������п����п���û�ͭ���ӣ����ˣ�����ΪCu���ܺ���Zn����ҺΪ����п������Ũ������ȴ�ᾧ�����˵õ�ZnSO4•7H2O���壬�Դ˽����⣮
��� �⣺����п����Ҫ�ɷ�ΪZnO������ZnSiO3��FeCO3��CuO�ȣ���ϡ�����ܽ⣬���ˣ���Һ�к�������п������ͭ�������������ټӸ��������Һ���������ӷ�Ӧ����MnO��OH��2�������������������ˣ���Һ�к�������ͭ������п����п���û�ͭ���ӣ����ˣ�����ΪCu���ܺ���Zn����ҺΪ����п������Ũ������ȴ�ᾧ�����˵õ�ZnSO4•7H2O���壬
��1�������������ᣬ��ϡ�����ֽ��ݣ����ˣ���Һ�к�������п������ͭ�������������õ�����AΪH2SiO3���ʴ�Ϊ��������ˣ�H2SiO3��
��2����PHԼΪ2.1����Һ�м��������أ����������ӷ�Ӧ����MnO��OH��2�����������������÷�Ӧ�����ӷ���ʽΪ3Fe2++MnO4-+8H2O=3Fe��OH��3��+MnO��OH��2��+5H+��
�ʴ�Ϊ��3Fe2++MnO4-+8H2O=3Fe��OH��3��+MnO��OH��2��+5H+��
��3��ZnSO4•7H2O���¶Ƚϸ�ʱ��ʧȥ�ᾧˮ�����ڼ�ѹ���������¸��
�ʴ�Ϊ�����ͺ�ɵ��¶ȣ���ֹZnSO4•7H2O�ֽ⣻
��4��28.70 g ZnSO4•7H2O�����ʵ���Ϊ0.1mol����ZnԪ���غ��֪������ZnSO4•H2O��ZnSO4��ZnO��Zn3O��SO4��2ʱ�����ʵ�����Ϊ0.1mol��
����ZnSO4•H2O������Ϊ17.90g��100�棩��
����ZnSO4������Ϊ16.10g��250�棩��
����Zn3O��SO4��2������Ϊ0.1mol��$\frac{1}{3}$��403=13.43g��680�棩��
����ZnO������Ϊ8.10g��930�棩��
����680��ʱ���ù���Ļ�ѧʽΪZn3O��SO4��2��
�ʴ�Ϊ��b��
���� ������ZnSO4•7H2O���Ʊ�Ϊ֪ʶ�����������˻���ʵ�������������ԭ��Ӧ����ѧ��Ӧ���ʵ�Ӱ�����ء���ѧ����ʽ����д��֪ʶ����Ŀ�Ѷ��еȣ������ڿ���ѧ���ķ���������ʵ��������

 ������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������T������������������������ȣ������жϲ���ȷ���ǣ�������
������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������T������������������������ȣ������жϲ���ȷ���ǣ�������| A�� | �����̬�⻯������ȶ��ԣ�R��Q | |
| B�� | ����������Ӧˮ��������ԣ�Q��W | |
| C�� | ԭ�Ӱ뾶��T��Q��R | |
| D�� | �����Ӱ뾶��T��R |
| A�� | ������ԭ���������� | B�� | ������ѹǿ���� | ||
| C�� | �����ڷ����������� | D�� | �����ķ�Ӧ����ȡ����Ӧ |


 +H2O��R��R��ΪH��������
+H2O��R��R��ΪH��������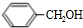 ��C�к��еĹ�����̼̼˫����ȩ����д���ƣ���
��C�к��еĹ�����̼̼˫����ȩ����д���ƣ��� ��
�� ��
��


 ȷ����Ӧ�Ļ�ѧ����ʽ���Ƶõ�MgO�Ĵ��ȣ�����MgSO410.0g��������ʯӢ���У�����ͼ����װ�ý������飮���鲽�����£�
ȷ����Ӧ�Ļ�ѧ����ʽ���Ƶõ�MgO�Ĵ��ȣ�����MgSO410.0g��������ʯӢ���У�����ͼ����װ�ý������飮���鲽�����£�
 �������ܷ������л���Ӧ�����Тܡ��ޣ�
�������ܷ������л���Ӧ�����Тܡ��ޣ�
 ��
�� ��
��
 ��
�� ��
�� ��
�� ��
��