��Ŀ����
����Ŀ�����������������ư�Ѫ����������Ч���л�������M(![]() )�Ǻϳ�����������м��壬��һ�ֺϳ�·����ͼ��
)�Ǻϳ�����������м��壬��һ�ֺϳ�·����ͼ��

��֪����������������ڴ��Ƶȼ������ϼ������·����������ã�ʧȥһ���Ӵ��õ��¡�ͪ�������磺
2CH3COOC2H5 CH3COCH2COOC2H5+C2H5OH
CH3COCH2COOC2H5+C2H5OH
�ش��������⣺
(1)�л���A�к��������ŵ�����Ϊ___��
(2)M�ķ����������___��̼ԭ����ͬһ��ƽ���ϣ�M����˳���칹�����䷴ʽ�칹��Ľṹ��ʽ��___��
(3)��Ӧ�ٵĻ�ѧ����ʽΪ___���䷴Ӧ����Ϊ___��
(4)�л���![]() �ж���ͬ���칹�壬���к��б�����������������___�֣�д������һ�־�������̼��ͬ���칹��Ľṹ��ʽΪ___��
�ж���ͬ���칹�壬���к��б�����������������___�֣�д������һ�־�������̼��ͬ���칹��Ľṹ��ʽΪ___��
(5)д�����Ҵ������ᡢ Ϊԭ�Ϻϳ�
Ϊԭ�Ϻϳ� �ĺϳ�·��(�����Լ���ѡ)___��
�ĺϳ�·��(�����Լ���ѡ)___��
���𰸡��Ȼ� 9 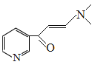
![]() +C2H5OH
+C2H5OH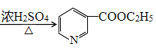 +H2O ������Ӧ(��ȡ����Ӧ) 14
+H2O ������Ӧ(��ȡ����Ӧ) 14 ![]()
��������
�������̷����ɵã�A��Ũ���������������C2H5OH����������Ӧ����![]() ����A�Ľṹ��ʽΪ
����A�Ľṹ��ʽΪ![]() ��
�� ![]() ���Ҵ���(C2H5ONa)��������CH3COOC2H5������֪��Ϣ�ķ�Ӧ����B�����B�ķ���ʽ���ɵ�B�Ľṹ��ʽΪ
���Ҵ���(C2H5ONa)��������CH3COOC2H5������֪��Ϣ�ķ�Ӧ����B�����B�ķ���ʽ���ɵ�B�Ľṹ��ʽΪ![]() ��B��HCl����ȡ����Ӧ����
��B��HCl����ȡ����Ӧ����![]() ��
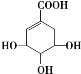
��![]() ��(CH3O)2CHN(CH3)2��Ӧ����M���ݴ˷������
��(CH3O)2CHN(CH3)2��Ӧ����M���ݴ˷������
(1)A�Ľṹ��ʽΪ![]() �����к��������ŵ�����Ϊ�Ȼ���
�����к��������ŵ�����Ϊ�Ȼ���
(2)�л�������M�Ľṹ��ʽΪ![]() ����̼̼˫��ֱ������������ԭ�ӹ��棬���ӽṹ�е���Ԫ���е�̼ԭ����ͬһƽ�棬̼��˫������Ԫ�����棬̼̼˫���ϵ�̼ԭ���뵪ԭ�ӵ�����������תһ���Ƕȵ�ԭ���ϵļ�����̼̼˫�����棬��÷����������9��̼ԭ����ͬһ��ƽ���ϣ�M����˳���칹��������Ԫ��֧���ϵ�̼̼˫�������γ��䷴ʽ�칹�壬�ṹ��ʽ��
����̼̼˫��ֱ������������ԭ�ӹ��棬���ӽṹ�е���Ԫ���е�̼ԭ����ͬһƽ�棬̼��˫������Ԫ�����棬̼̼˫���ϵ�̼ԭ���뵪ԭ�ӵ�����������תһ���Ƕȵ�ԭ���ϵļ�����̼̼˫�����棬��÷����������9��̼ԭ����ͬһ��ƽ���ϣ�M����˳���칹��������Ԫ��֧���ϵ�̼̼˫�������γ��䷴ʽ�칹�壬�ṹ��ʽ�� ��
��
(3)��Ӧ��ΪA��Ũ���������������C2H5OH����������Ӧ����![]() ����ѧ����ʽΪ
����ѧ����ʽΪ![]() +C2H5OH
+C2H5OH +H2O���䷴Ӧ����Ϊ������Ӧ(��ȡ����Ӧ)��
+H2O���䷴Ӧ����Ϊ������Ӧ(��ȡ����Ӧ)��
(4)�л���![]() �ж���ͬ���칹�壬���к��б����������������ṹΪ������- C2H5��-NO2��ϣ����ڼ��3�ֽṹ������-NO2������-CH3��ϣ���6�ֽṹ������-CH3��-CH2NO2��ϣ����ڼ��3�ֽṹ������-CH2CH2NO2��ϣ���1�ֽṹ������-CH(CH3)NO2��ϣ���1�ֽṹ������14��ͬ���칹�壻����һ�־�������̼��ͬ���칹��Ľṹ��ʽΪ
�ж���ͬ���칹�壬���к��б����������������ṹΪ������- C2H5��-NO2��ϣ����ڼ��3�ֽṹ������-NO2������-CH3��ϣ���6�ֽṹ������-CH3��-CH2NO2��ϣ����ڼ��3�ֽṹ������-CH2CH2NO2��ϣ���1�ֽṹ������-CH(CH3)NO2��ϣ���1�ֽṹ������14��ͬ���칹�壻����һ�־�������̼��ͬ���칹��Ľṹ��ʽΪ![]() ��
��
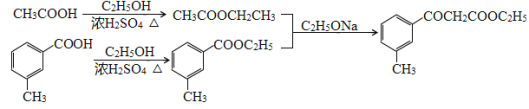
(5)���Ҵ������ᡢ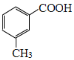 Ϊԭ�Ϻϳ�
Ϊԭ�Ϻϳ�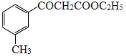 ���Ҵ���������Ũ���������������������������
���Ҵ���������Ũ���������������������������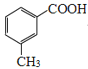 ���Ҵ���Ũ�����������������
���Ҵ���Ũ�����������������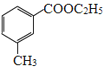 ��
�� �������������Ҵ��������·�����֪��Ϣ�ķ�Ӧ����
�������������Ҵ��������·�����֪��Ϣ�ķ�Ӧ����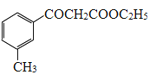 ���ϳ�·��Ϊ��
���ϳ�·��Ϊ�� ��
��

 ��У����ϵ�д�
��У����ϵ�д�