��Ŀ����
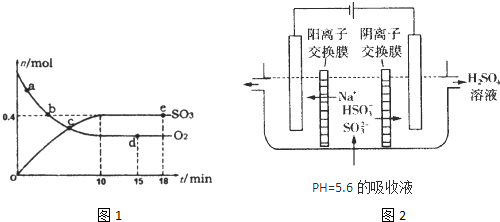
��400�棬1.01��105Paʱ����SO2��14molO2 ѹ��һ��װ�д�����V���ܱշ�Ӧ���У�����2SO2+O2 2SO3�ķ�Ӧ��������10min��ﵽƽ��ʱ�������������2molSO2��12molO2:
2SO3�ķ�Ӧ��������10min��ﵽƽ��ʱ�������������2molSO2��12molO2:
��1��SO2����ʼŨ��Ϊ_______��SO3��������ƽ��Ũ��Ϊ_________��
��2��SO2��ת����Ϊ________��O2��ƽ����Ӧ����Ϊ________��
��3��ƽ��ʱSO3������ռ������İٷ���Ϊ__________����ʱ��ϵѹǿΪ��Ӧ��ʼʱ��ϵѹǿ��________����
��4����ƽ����ټ���2molSO2��12molO2��4molSO3(��)����ʱ����Ӧ���ʽ�_______���淴Ӧ���ʽ�_______��ƽ��_______�ƶ���
 2SO3�ķ�Ӧ��������10min��ﵽƽ��ʱ�������������2molSO2��12molO2:
2SO3�ķ�Ӧ��������10min��ﵽƽ��ʱ�������������2molSO2��12molO2: ��1��SO2����ʼŨ��Ϊ_______��SO3��������ƽ��Ũ��Ϊ_________��
��2��SO2��ת����Ϊ________��O2��ƽ����Ӧ����Ϊ________��
��3��ƽ��ʱSO3������ռ������İٷ���Ϊ__________����ʱ��ϵѹǿΪ��Ӧ��ʼʱ��ϵѹǿ��________����
��4����ƽ����ټ���2molSO2��12molO2��4molSO3(��)����ʱ����Ӧ���ʽ�_______���淴Ӧ���ʽ�_______��ƽ��_______�ƶ���
(1)6/Vmol��L-1 ��4/Vmol��L-1
(2)66.67% ��0.2/Vmol��L-1��min-1
(3)22.22% ��0.9��
(4)������������(������Ӧ����)
(2)66.67% ��0.2/Vmol��L-1��min-1
(3)22.22% ��0.9��
(4)������������(������Ӧ����)

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
�����Ŀ