��Ŀ����
10��ijѧ����0.2000mol•L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�������������ˮϴ�Ӽ�ʽ�ζ��ܣ�ע��0.2000mol•L-1�ı�NaOH��Һ����0���̶�������
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ�壻
�۵���Һ������0����0���̶������£������¶�����
����ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ��
���ñ�Һ�ζ����յ㣬���µζ���Һ�������
�������ϵζ�����2-3�Σ�
��ش�
��1�����ϲ����д�����ǣ����ţ��٣��ô�������ᵼ�²ⶨ���ƫ�� ���ƫ����ƫС������Ӱ�족����
��2��������У���ȡ20.00mL����ҺӦʹ����ʽ�ζ��ܣ����������ƣ�������ƿװҺǰ��������������ˮ���ⶨ�����Ӱ�죨�����ƫС������Ӱ�족����
��3������ݵζ�ʱ�۾�Ӧע����ƿ����Һ��ɫ�仯���жϵ���ζ��յ�������ǣ���ƿ����Һ����ɫ��Ϊdz��ɫ������Ӳ���ɫ��
��4��������ʵ�����ݼ�¼��
| �ζ����� | ���������mL�� | NaOH��Һ���������mL�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.00 | 18.10 |
| 2 | 20.00 | 0.00 | 16.30 |
| 3 | 20.00 | 0.00 | 16.22 |
A���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
B����ƿ�ô���Һ��ϴ
C��NaOH��Һ����ʱ��������в��ֱ���
D���ζ�����ʱ�����Ӷ���
��5�������ϱ���¼���ݣ�ͨ������ɵã�������Ũ��Ϊ��0.1626mol•L-1��
���� ��1�����ݼ�ʽ�ζ�����װҺǰӦ�ô�װҺ������ϴ������c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��жϣ�
��2�����ݾ�ȷ��ȡҺ�������������õζ��ܣ�����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��жϣ�
��3�������к͵ζ��У��۾�Ӧע�ӵ�����ƿ����Һ��ɫ�仯��������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��4������c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��жϣ�
��5���ȸ������ݵ���Ч�ԣ���ȥ��1�����ݣ�Ȼ�����2��3��ƽ������V��NaOH�������Ÿ���NaOH��HCl�����㣮
��� �⣺��1����ʽ�ζ�����װҺǰӦ�ô�װҺ������ϴ��������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶������ϣ���ʽ�ζ���δ�ñ�NaOH��Һ��ϴ��ֱ��ע���NaOH��Һ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$������c������ƫ��
�ʴ�Ϊ���٣�ƫ��
��2����������ԣ���ȡ20.00mL����ҺӦʹ����ʽ�ζ��ܣ�����ƿװҺǰ��������������ˮ��V���������䣬����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$������c���������䣻
�ʴ�Ϊ����ʽ�ζ��ܣ���Ӱ�죻
��3���к͵ζ��У��۾�Ӧע�ӵ�����ƿ����Һ��ɫ�仯���ζ�ʱ������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣬���Ե��������һ��NaOH��Һ����Һ����ɫ��Ϊ�ۺ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ����ƿ����Һ��ɫ�仯����ƿ����Һ����ɫ��Ϊdz��ɫ������Ӳ���ɫ��
��4�����ϱ����Կ�������1�εζ���¼��NaOH��Һ������Զ��ں����ε��������õ�����Ũ��ƫ��
A���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������ݣ���������������Һ���ƫ����������Ũ��ƫ��A��ȷ��
B����ƿ�ô���Һ��ϴ������Һ�����ʵ���ƫ��������������Һ���ƫ����������Ũ��ƫ��B��ȷ
C����NaOH��Һ����ʱ��������в��ֱ��ʣ�Ũ�Ƚ��ͣ����÷�̪Ϊָʾ�������ղ��ﲻ�䣬û��Ӱ�죬��C����
D���ζ�����ʱ�����Ӽ�������������������Һ���ƫС����������Ũ��ƫС����D����
��ѡ��AB��
��5�����εζ����ĵ����Ϊ��18.10mL��16.30mL��16.22����ȥ��1�����ݣ�Ȼ�����2��3��ƽ������V��NaOH��=16.26mL��
NaOH��HCl
1 1
0.2000mol•L-1��16.26mL c��HCl����20.00mL
��ã�c��HCl��=0.1626 mol•L-1��
�ʴ�Ϊ��0.1626��
���� ���⿼���к͵ζ�ʵ�顢�ζ��ܽṹ��ʹ�á��������Լ�����ȣ��ѶȲ���ע�������к͵ζ���ԭ����ζ��ܵĽṹ�����ȣ�

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�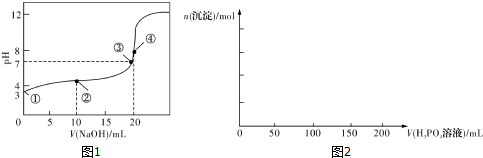
��2������Na2SO3��Һ����SO2�Ĺ����У�pH��n��SO32-����n��HSO3-���仯��ϵ���±���
| n��SO32-����n��HSO3-�� | 1��9 | 1��1 | 1��91 |
| pH | 8.2 | 7.2 | 6.2 |
��3����0.1mol•L-1��NaHSO3��ͨ�˰�������Һ������ʱ����Һ�е�c��H+����c��OH-����c��SO32-����c��Na+����c��NH4+������������Ũ�ȴ�С��ϵ��c��Na+����c��SO32-����c��NH4+����c��H+��=c��OH-����
��4����֪Ca3��PO4��2��CaHPO4��������ˮ����Ca��H2PO4��2���ܣ��ں�0.1molCa��OH��2�ij���ʯ��ˮ����μ���1mol•L-1��H3PO4������ͼ2���������ɳ��������ʵ�����H3PO4���������0��ʼ��200mL��ͼ��
��ѧ��ȤС��ȡ20.00mL����ϡ���������ƿ�У����μ�2��3�η�̪��Һ��ָʾ������0.2000mol•L-1�ı�NaOH��Һ���еζ����ظ������ζ�����3�Σ�����¼���ݣ�
��1���ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�B��������ţ�
A���ζ�����Һ��ı仯 B����ƿ����Һ��ɫ�ı仯
��2���жϵζ��յ�������ǣ���Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
��3�������������ݣ���������������Һ��Ũ��Ϊ0.2000mol/L��
| �ζ����� | ����Һ�����mL�� | ��NaOH��Һ������¼��mL�� | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 20.00 | 0.40 | 20.40 |
| �ڶ��� | 20.00 | 4.00 | 24.00 |
| ������ | 20.00 | 2.00 | 24.10 |
��ȡˮ��10.0ml����ƿ�У�����10.0ml��KI��Һ��������������ָʾ��2��3�Σ�
�ڽ��Լ����Ƶ�0.01mol•L-1��Na2S2O3��Һװ��ζ����У�����Һ�棬���¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2+2Na2S2O3=2NaI+2Na2S4O6���Իش������ʴ�
��1������ٷ����Ļ�ѧ��Ӧ����ʽΪ��Cl2+KI=I2+2KCl�������ָʾ���ǵ�����Һ��
��2�������Ӧʹ�ü�ʽ�ζ��ܣ�
��3���ȵ�����Ũ�ȱ�ʵ��Ũ�Ȼ�ƫ���������ԭ�������ACE������ţ�
A ���Ʊ�Na2S2O3��Һ����ʱ����ˮ�����̶���
B ��ƿˮϴ��ֱ��װ����ˮ��
C װ��Na2S2O3��Һ�ĵζ���ˮϴ��û����ϴ
D �ζ������յ�ʱ�����Ӷ����ζ��ܶ�����
E �ζ�ǰ���첿�������ݣ��ζ�����ʧ
�����ζ����õ�ָʾ����������һ�ֳ���������֪һЩ���ε���ɫ��Ksp��20�棩���±����ⶨˮ�����Ȼ���ĺ��������ñ���������Һ���еζ���
| ��ѧʽ | AgCl | AgBr | AgI | Ag2S | Ag2CrO4 |
| ��ɫ | ��ɫ | dz��ɫ | ��ɫ | ��ɫ | ��ɫ |
| Ksp | 2.0��10-10 | 5.4��10-13 | 8.3��10-17 | 2.0��10-48 | 2.0��10-12 |
A��KBrB��KIC��K2S D��K2CrO4
��2����BaCl2��Һ�м���AgNO3��KBr�������ֳ�������ʱ��$\frac{c��Br-��}{c��Cl-��}$=2.7��10-3��
| A�� | SiO2��CsCl��CBr4��CF4 | B�� | CF4��CCl4��CBr4��Cl4 | ||
| C�� | ���ʯ������裾�������裾̼���� | D�� | NaF��MgF2��AlF3 |
 ����к͵ζ�����ѧ��ѧ����Ҫ�Ķ���ʵ��֮һ��ij�о���ѧϰС��ȷ����������ʵ�飬��ȡ1.00g�����Ŀ�������Ʒ���250ml��Һ��ȡ��10.00ml������֪Ũ��Ϊ0.040mol•L-1��������еζ������ʲ������ᷴӦ����
����к͵ζ�����ѧ��ѧ����Ҫ�Ķ���ʵ��֮һ��ij�о���ѧϰС��ȷ����������ʵ�飬��ȡ1.00g�����Ŀ�������Ʒ���250ml��Һ��ȡ��10.00ml������֪Ũ��Ϊ0.040mol•L-1��������еζ������ʲ������ᷴӦ��������Ҫ��ش��������⣺
��1������250mL 0.040mol•L-1��������Һʱ��Ҫ�õ��IJ�����������Ͳ���ձ�������������ͷ�ιܺ�250mL����ƿ��
��2��Ϊ�ⶨ�ÿ�������Ʒ�Ĵ��ȣ����εζ����������������£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����������Һ�������mL�� | 20.05 | 20.00 | 22.10 | 19.95 |
��3��������������δ��������ϴ�ζ��ܣ���ⶨ�����ƫ���ƫ����ƫС������Ӱ�족����
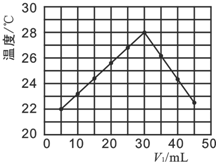
��4������һ��ʵ���У��о���С�齫V1 mL 1.0mol•L-1 HCl��Һ��V2 mL δ֪Ũ�ȵ�NaOH��Һ���Ȼ�Ϻ�������¼��Һ�¶ȣ�ʵ������ͼ��ʾ��ʵ����ʼ�ձ���V1+V2=50mL����
������������ȷ����b��
a����ʵ��Ļ����¶�Ϊ22��
b����V1=40ʱ����Һ��c��Na+����c��Cl-��
c��NaOH��Һ��Ũ��Ϊ1.0mol•L-1
d�����������������䣬ֻ��HCl��ΪCH3COOH����ʵ�飬Ҳ�õ���ͼ��ʵ������
| A�� | һ�������£���1 mol N2��3 mol H2��ϣ���ַ�Ӧ��ת�Ƶĵ�����Ϊ6 NA | |
| B�� | 1.5 mol NO2������ˮ��Ӧ��ת�Ƶĵ�����Ϊ1.5 NA | |
| C�� | 6.4 g��S2��S4��S8��ɵĻ���ﺬ��ԭ����Ϊ0.2 NA | |
| D�� | ���³�ѹ�£�11.2 L Cl2����ԭ����ΪNA |
| A�� | �ܽ�Ͻ�ʱ�ռ���NO�������Ϊ2.24L���ڱ�״���� | |
| B�� | ����Ͻ������������Ϊ9.6g | |
| C�� | ������ȫʱ����NaOH��Һ�����Ϊ150mL | |
| D�� | �μӷ�Ӧ����������ʵ���Ϊ0.4mol |
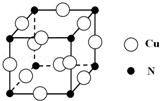 ��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺
��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺ ��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3��
��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3��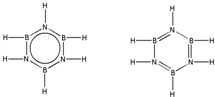 ��
��