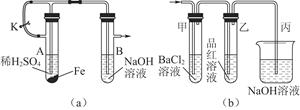
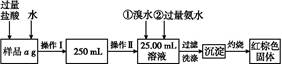
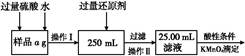
��Ŀ����
����������������(��Ҫ�ɷ�ΪFe2O3��FeO��SiO2��)Ϊԭ���Ʊ��ߵ���������(Fe2O3)�����������������£�

�Իش��������⣺
(1)��ҺX�к��еĽ�����������______________(�����ӷ���)��
(2)������п�ѡ��________������Һ��pH(����ĸ)��
(3)������У�FeCO3������ȫ����Һ�к�������Fe2��������Fe2���ķ�����________________________________��
(4)������ķ�Ӧ�¶�һ���������35�����£���Ŀ����_____________________________________________��
(5)�ڿ���������FeCO3���ɲ�Ʒ�������Ļ�ѧ����ʽΪ______________________________��

�Իش��������⣺
(1)��ҺX�к��еĽ�����������______________(�����ӷ���)��
(2)������п�ѡ��________������Һ��pH(����ĸ)��
| A��ϡ���� | B����ˮ | C������������Һ | D�����������Һ |
(4)������ķ�Ӧ�¶�һ���������35�����£���Ŀ����_____________________________________________��
(5)�ڿ���������FeCO3���ɲ�Ʒ�������Ļ�ѧ����ʽΪ______________________________��
(1)Fe2����Fe3����(2)B
(3)ȡ������Һ���������軯����Һ�����Ժ�ɫ��Ȼ��μ���ˮ����Һ��Ϊ��ɫ
(4)��ֹNH4HCO3�ֽ⣬����Fe2��ˮ��
(5)4FeCO3��O2 2Fe2O3��4CO2
2Fe2O3��4CO2
(3)ȡ������Һ���������軯����Һ�����Ժ�ɫ��Ȼ��μ���ˮ����Һ��Ϊ��ɫ
(4)��ֹNH4HCO3�ֽ⣬����Fe2��ˮ��
(5)4FeCO3��O2
 2Fe2O3��4CO2
2Fe2O3��4CO2(1)����������(��Ҫ�ɷ�ΪFe2O3��FeO��SiO2��)�������ˣ�SiO2Ϊ��������Һ�к��еĽ�����������Fe2����Fe3����(2)�����Ҫ��pH���ߣ��������Ҫ��NH4HCO3����ʹ�ð�ˮ����ʹ������������Һ����Ʒ��������Na��������ѡB��(4)NH4HCO3�����ֽ⣬���»�ٽ�Fe2��ˮ�⡣

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 ��
�� 


 E(SCN)3��
E(SCN)3��