��Ŀ����
16�� ��Al��NiO��OH��Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ�ͨ�����ԭ�����������Է�ˮ�е�NO3-������˵��������ǣ�������
��Al��NiO��OH��Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ�ͨ�����ԭ�����������Է�ˮ�е�NO3-������˵��������ǣ�������| A�� | �����͵�ع���ʱ�������ĵ缫��Ӧʽ��Al+4OH--3e-�TAlO2-+2H2O | |
| B�� | Ϊ��ǿ��Һ�ĵ����ԣ�I��ˮ�пɼ�������Na2SO4 | |
| C�� | AΪ��Դ������H+�Ӣ���������� | |
| D�� | ������ӦʽΪ��2NO3-+6H2O+10e-�TN2��+12OH- |
���� A������NaOH��Һ�Ĵ��ڣ�Al����������NaAlO2��
B����������Na2S04���壬��ʹ�������ƶ�������Ŀ���࣬���Dz���Ӱ��缫��Ӧ��
C�������ʱ������������������
D����������������ӵõ��ӷ�����ԭ��Ӧ��
��� �⣺A�������͵�طŵ�ʱ���ؼ��ж�Al�������������NaOH��Һ�Ĵ��ڣ�Al����������NaAlO2������Al��OH��3����ΪA1+4OH--3e-=AlO2-+2H2O����A��ȷ��
B�������ڹ�����OH-�ŵ磬����Ϊ��ǿ��Һ�ĵ����ԣ�I��ˮ�пɼ�������Na2S04���壬��B��ȷ��
C��AΪ��Դ�����������ʱ����������������Ӧ��2H2O-4e-=4H++O2����H+�Ӣ��������������C��ȷ��
D����������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2NO3-+12H++10e-=N2��+6H2O����D����
��ѡD��
���� ���⿼��ѧ�����ε�صĹ���ԭ���Լ��缫��Ӧʽ����д��Ӧ��֪ʶ��ע��֪ʶ�Ĺ��ɺ������ǹؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
6����ijNaOH��Һ��ȡ�����������Һʱ������������һ��û�з����仯���ǣ�������
| A�� | ��Һ��NaOH�����ʵ��� | B�� | ��Һ��Na+�����ʵ���Ũ�� | ||
| C�� | ��Һ��Na+����Ŀ | D�� | ��Һ������ |
7���������ʵij��ӷ�����ȷ���ǣ�������
| ѡ�� | ���ᴿ������ | ���� | �����Լ� | ���ӷ��� |
| A | CO2 ��g�� | SO2��g�� | ����Na2CO3��Һ��ŨH2SO4 | ϴ�� |
| B | NH4Cl��aq�� | Fe3+��aq�� | NaOH��Һ | ���� |
| C | NaCl��s�� | KNO3��s�� | AgNO3��Һ | ���� |
| D | Cu��s�� | Ag��s�� | CuSO4��Һ | ��ⷨ |
| A�� | A | B�� | B | C�� | C | D�� | D |
11������˵����ȷ���ǣ�������
| A�� | ����CCl4��˫��ˮ������Һ���Ƿ���I- | |
| B�� | ����Ư������������ˮ����Na2S03��Һ | |
| C�� | ����KI������Һ����NO2�������� | |
| D�� | ����ˮ����NO���� |
1���������ӷ���ʽ���Ȼ�ѧ����ʽ��ȷ���ǣ�������
| A�� | �ڲ�ͬ�¶��£�����ͬŨ����ͬ�����Na2S2O3��H2SO4��Ӧ��̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죺3S2O32-+2SO42+10H+�T6SO2��+2S��+5H2O | |
| B�� | ���������£�KI��O2���������ӷ���ʽ��4I-+O2+4H+�T2I2+2H2O | |
| C�� | ��֪25�桢101kPaʱ��1molH2����������ȫ��Ӧ������̬HBr�ų�����Q kJ�����Ȼ�ѧ����ʽΪH2��g��+Br2��g���T2HBr��g����H=-2Q kJ•mol-1 | |
| D�� | ��ʾ�к��ȵ��Ȼ�ѧ����ʽ��2HCl��aq��+Ba��OH��2��aq���TBaCl2��aq��+2H2O��l����H=-114.6 |
8��һ������½����ȶ������������ۼ�����Ҫԭ���ǣ�������
| A�� | ����������������������Ӷ���ͬ�ֵ�� | |
| B�� | �����в����˶� | |
| C�� | ��������ֱ����1��100nm֮�䣬����С�������������� | |
| D�� | ������������ |
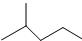 ��ʾ�ķ���ʽC6H14��������2-�����飬�˴Ź�����������5��壮
��ʾ�ķ���ʽC6H14��������2-�����飬�˴Ź�����������5��壮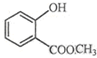 �к��еĹ����ŵ�����Ϊ�ǻ���������
�к��еĹ����ŵ�����Ϊ�ǻ���������
 ��
�� ��
��