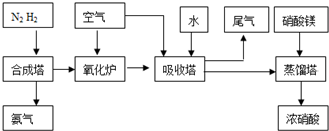
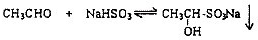��Ŀ����
��2009?��ɽģ�⣩ij�о���ѧϰС��ѧϰ�˹�ҵ�������Ƽ����ԭ����
[�������]�ܷ���ʵ����ģ�⡰�����Ƽ������ȡNaHCO3�Ĺ����أ�
[ʵ��ԭ��]д�������Ƽ��Ӧ�Ļ�ѧ����ʽΪ
��ش��������⣺
��1������Aװ�������Եķ����ǣ�����������©�����������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ������Թ��ڵ�ˮ�棬ֹͣ��ˮ����
��2��D��������װ��A��װ��C֮������徻��װ�ã���������
��3��ʵ��ʱ����NaCl��Һ��ͨ��϶��
��ʹCO2���ױ����� ��NH3��CO2������ȡ ��CO2���ܶȱ�NH3��
��4����
���Ҫ�Ƶô�����跢���ķ�Ӧ�ǣ�д����Ӧ�Ļ�ѧ����ʽ����
[�ó�����]���á������Ƽ����ʵ���ҿ�����ȡNaHCO3��
[�������]�ܷ���ʵ����ģ�⡰�����Ƽ������ȡNaHCO3�Ĺ����أ�
[ʵ��ԭ��]д�������Ƽ��Ӧ�Ļ�ѧ����ʽΪ
NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl
NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl
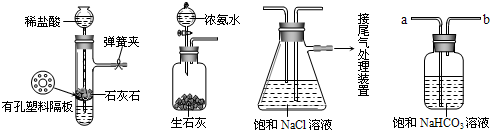
��[ʵ����֤]��ͼ�Ǹ�ѧϰС�����ģ��ʵ��ʱ���õ��IJ�����Ҫװ�ã�
��ش��������⣺
��1������Aװ�������Եķ����ǣ�����������©�����������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ������Թ��ڵ�ˮ�棬ֹͣ��ˮ����
©�������Թ��е�Һ��ˮ�����ֲ��ٱ仯��©���е�Һ��ˮ���治���½���
©�������Թ��е�Һ��ˮ�����ֲ��ٱ仯��©���е�Һ��ˮ���治���½���
��˵��װ�ò�©������2��D��������װ��A��װ��C֮������徻��װ�ã���������
a
a
����a��b����D�������dz�ȥHCl
HCl
���壮�ɷ�ƿ���Լ���Ϊ̼������Һ��
��
����ɡ���������3��ʵ��ʱ����NaCl��Һ��ͨ��϶��
NH3
NH3
����ͨ��������CO2
CO2
����ԭ������
��
������д��ţ���ʹCO2���ױ����� ��NH3��CO2������ȡ ��CO2���ܶȱ�NH3��
��4����
����
����
�ķ��������ɵ�NaHCO3����ӻ�����з�����������Ҫ�Ƶô�����跢���ķ�Ӧ�ǣ�д����Ӧ�Ļ�ѧ����ʽ����
2NaHCO3
Na2CO3+H2O+CO2��
| ||
2NaHCO3
Na2CO3+H2O+CO2��
��
| ||
[�ó�����]���á������Ƽ����ʵ���ҿ�����ȡNaHCO3��
��������1��ע��һ������ˮ���Թ������屻ѹ������ѹǿ����ʹ�ó���Һ��Һ���ֲ���ʱ��˵��װ�ò�©����
��2��Ϊʹ����ͨ����Һ�������aͨ����Һ�����������е�HCl����Һ�е�̼�����Ʒ�Ӧ�������գ�����̼������Һ��������̼���屻̼������Һ���գ�
��3��������̼��ˮ��Ӧ�γɲ��ȶ���̼�ᣬ��ʹˮ���ն�����̼�����٣����Ѷ�����̼ͨ�����а������ʼ��Ե�ˮ�У���ʹ���ɵ�̼���백ˮ������Ӧ�������������̼��������գ�
��4���ѹ�����Һ�����γɻ�����й����������ķ���Ϊ���ˣ�
��2��Ϊʹ����ͨ����Һ�������aͨ����Һ�����������е�HCl����Һ�е�̼�����Ʒ�Ӧ�������գ�����̼������Һ��������̼���屻̼������Һ���գ�
��3��������̼��ˮ��Ӧ�γɲ��ȶ���̼�ᣬ��ʹˮ���ն�����̼�����٣����Ѷ�����̼ͨ�����а������ʼ��Ե�ˮ�У���ʹ���ɵ�̼���백ˮ������Ӧ�������������̼��������գ�
��4���ѹ�����Һ�����γɻ�����й����������ķ���Ϊ���ˣ�
����⣺�����Ƽ���ö�����̼ͨ�백���ı����Ȼ�����Һ�з�Ӧ����̼�����ƾ��壬��Ӧ�Ļ�ѧ����ʽ��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��
�ʴ�Ϊ��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl
��1����ˮ��ע����ڼ�ѹ����©����Һ�岻���������Թܣ�ʹ����©�������Һ�����Һ������Һ���ֲ��䣬˵��װ�����������ã�
�ʴ�Ϊ������©����ע��ˮ��©�������Թ��е�Һ��ˮ�����ֲ��ٱ仯��©���е�Һ��ˮ���治���½���
��2�������a��ͨ��ʱ����ʹ����ͨ����Һ���ﵽ���������Ŀ�ģ�װ��D��ʢ�ŵ�̼��������Һ������������е�HCl������Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���Ӷ���ȥ���������е�HCl��������̼������Һ��������̼Ҳ�ᱻ���գ�
�ʴ�Ϊ��a���Ȼ��⣨��HCl������
��3����ͨ�백����������ˮ�γɳʼ��Եİ�ˮ���������̼��ˮ���ɵ�̼�ᷢ����Ӧ���������ڶ�����̼��������գ�
�ʴ�Ϊ��NH3��CO2���٣�
��4��ͨ�����ˣ��ɰѻ�����Һ�е�̼�����ƾ���������������Ƶô�����Ҫ��̼�����ƹ�����ȷֽ�õ�����Ӧ��ѧ����ʽΪ��2NaHCO3
Na2CO3+H2O+CO2��
�ʴ�Ϊ�����ˣ�2NaHCO3
Na2CO3+H2O+CO2��
�ʴ�Ϊ��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl
��1����ˮ��ע����ڼ�ѹ����©����Һ�岻���������Թܣ�ʹ����©�������Һ�����Һ������Һ���ֲ��䣬˵��װ�����������ã�
�ʴ�Ϊ������©����ע��ˮ��©�������Թ��е�Һ��ˮ�����ֲ��ٱ仯��©���е�Һ��ˮ���治���½���
��2�������a��ͨ��ʱ����ʹ����ͨ����Һ���ﵽ���������Ŀ�ģ�װ��D��ʢ�ŵ�̼��������Һ������������е�HCl������Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���Ӷ���ȥ���������е�HCl��������̼������Һ��������̼Ҳ�ᱻ���գ�
�ʴ�Ϊ��a���Ȼ��⣨��HCl������
��3����ͨ�백����������ˮ�γɳʼ��Եİ�ˮ���������̼��ˮ���ɵ�̼�ᷢ����Ӧ���������ڶ�����̼��������գ�
�ʴ�Ϊ��NH3��CO2���٣�
��4��ͨ�����ˣ��ɰѻ�����Һ�е�̼�����ƾ���������������Ƶô�����Ҫ��̼�����ƹ�����ȷֽ�õ�����Ӧ��ѧ����ʽΪ��2NaHCO3
| ||
�ʴ�Ϊ�����ˣ�2NaHCO3
| ||
���������⿼�������ù�ҵ�Ƽ�ԭ�������ʵ������ȡ̼���Ƶ�ʵ�鷽�������жϣ�����װ���е�ҩƷ�����ʼ�װ�õ����ӷ�ʽ����ȷ�жϻ�����װ�����õ�һ����Ч������

��ϰ��ϵ�д�
�����Ŀ
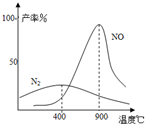 ��2009?��ɽģ�⣩��ҵ�ϳɰ����Ʊ�����һ��������������������£�
��2009?��ɽģ�⣩��ҵ�ϳɰ����Ʊ�����һ��������������������£�