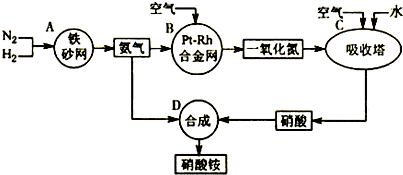
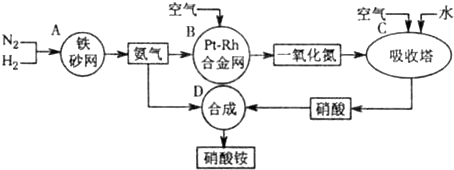
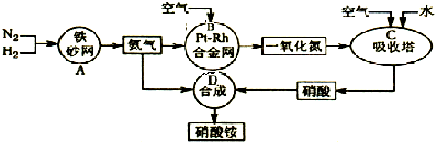
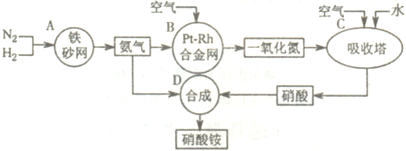
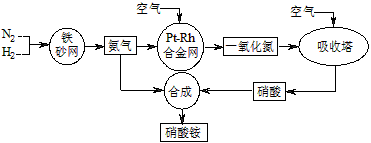
��Ŀ����
��ҵ��������淋�����ͼ����ͼ����ش�
��1����֪��N2(g)+3H2(g) 2NH3(g) ��H����92 kJ��mol-1��
2NH3(g) ��H����92 kJ��mol-1��
����500�桢2��02��107Pa��������������һ�ܱ������г���1molN2��3molH2����ַ�Ӧ�ų�������______(�<����>����=��)92��4kJ��������______________________________��
��Ϊ��Ч���������ת���ʣ�ʵ���������˲�ȡ�Ĵ�ʩ��____________
A�������¶� B�����ʺϴ������Ե��ʵ����� C������ѹǿ
D������ѹǿ E��ѭ�����úͲ��ϲ��䵪�� F����ʱ�Ƴ���
��2�� ��֪����Ͻ���δԤ��Ҳ�ᷢ�ȡ�д�����������Ļ�ѧ����ʽ��________________________���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK=________________�����¶�����ʱ��Kֵ______�����������С������Ӱ�족����
��3����һ���¶Ⱥ�ѹǿ���ܱ������У���ƽ����Է�������Ϊ8��5��H2��N2��ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������ƽ����Է�������Ϊ10��������ʱH2��ת���ʣ�д��������̣�
___________________________________________
 2NH3(g) ��H����92 kJ��mol-1��
2NH3(g) ��H����92 kJ��mol-1�� ����500�桢2��02��107Pa��������������һ�ܱ������г���1molN2��3molH2����ַ�Ӧ�ų�������______(�<����>����=��)92��4kJ��������______________________________��
��Ϊ��Ч���������ת���ʣ�ʵ���������˲�ȡ�Ĵ�ʩ��____________
A�������¶� B�����ʺϴ������Ե��ʵ����� C������ѹǿ
D������ѹǿ E��ѭ�����úͲ��ϲ��䵪�� F����ʱ�Ƴ���
��2�� ��֪����Ͻ���δԤ��Ҳ�ᷢ�ȡ�д�����������Ļ�ѧ����ʽ��________________________���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK=________________�����¶�����ʱ��Kֵ______�����������С������Ӱ�족����
��3����һ���¶Ⱥ�ѹǿ���ܱ������У���ƽ����Է�������Ϊ8��5��H2��N2��ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������ƽ����Է�������Ϊ10��������ʱH2��ת���ʣ�д��������̣�
___________________________________________
��1���٣�����101KPa��298K�����£�1mol������3mol������ȫ��Ӧ����2mol�������ų�92��4kJ�������÷�ӦΪ���淴Ӧ����Ӧ�ﲻ��ȫ����Ϊ���������Ϊ��Ӧ�¶�Ϊ500�棬���Էų�������С��92.4kJ
��CEF
��2��4NH3+5O2 4NO+6H2O�� K=
4NO+6H2O�� K=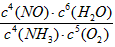 ����С
����С
��3���������������Ϊ1mol�����������ʵ���Ϊx�������������ʵ���Ϊ��1-x����
���У� 28x+2(1-x)=8��5������ʮ�ֽ��淨��
��ã�N2��x=0��25mol H2��1mol-0��25mol=0��75mol
����ƽ��ʱN2ת�������ʵ���Ϊy����
��CEF
��2��4NH3+5O2
 4NO+6H2O�� K=
4NO+6H2O�� K=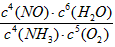 ����С
����С��3���������������Ϊ1mol�����������ʵ���Ϊx�������������ʵ���Ϊ��1-x����
���У� 28x+2(1-x)=8��5������ʮ�ֽ��淨��
��ã�N2��x=0��25mol H2��1mol-0��25mol=0��75mol
����ƽ��ʱN2ת�������ʵ���Ϊy����
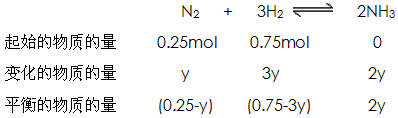
����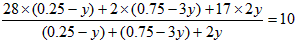
��ã�y=0��075mol
��������ת����Ϊ��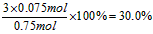
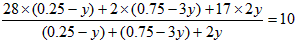
��ã�y=0��075mol
��������ת����Ϊ��
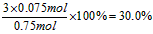

��ϰ��ϵ�д�
 ����5��2���ϵ�д�
����5��2���ϵ�д�
�����Ŀ