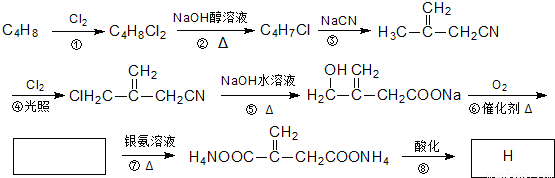
��Ŀ����
FeCl3������ӡˢ��·ͭ�帯ʴ��������ֹѪ����
��1����Ҫ�ܽ��·����3.2g��ͭ����������Ҫ1 mol��L��1 FeCl3��Һ�����Ϊ mL��
��2�����鸯ʴ��·ͭ������Һ���Ƿ����Fe3+���Լ��� ��
��3����ʴ��·ͭ������Һ��ͭԪ�غ����IJⶨ��
ȡ20.00mL��ʴ��·ͭ������Һ�ڵ���ƿ�У��ȼ�����NaF�������ķ�ӦΪFe3++6F����[FeF6]3�������ټ�������10%KI��Һ��ҡ�ȡ����ϵ���ƿƿ�������ڰ���5min����ַ�Ӧ����CuI�������ɣ����Ӽ��ε�����Һ����0.1000 mol��L?1Na2S2O3����Һ�ζ����յ�ʱ��������20.00mL��Һ���ⶨ�������й����ʵ�ת����ϵ���£�
���ⶨ�����е���ƿ�������ڰ���5min���ᵼ�²ⶨ��� �����ƫ�ߡ�����ƫ�͡�������Ӱ�족����
����ø�ʴҺ��ͭԪ�صĺ�������g��L?1��ʾ����д��������̡�
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д���������ʵ��������������õ��Ľ�����ȷ���ǣ� ��
ѡ�� | ʵ����������� | ���� |
A | ��1mL���������ֱ����6mLͬŨ�ȵ�NaOH��Һ��ϡH2SO4�У�ˮԡ������ͬʱ�����Һ��������ȫ��ʧ���������� | ˵������NaOH��Һ�е�ˮ��̶ȴ��� H2SO4��Һ�� |
B | ��10mL0.2mol��L?1NaOH��Һ�е���2��0.1 mol��L?1MgCl2��Һ��������ɫ�������ٵμ�2��0.1 mol��L?1FeCl3��Һ�����ɺ��ɫ���� | Ksp[Mg��OH��2]>Ksp[Fe��OH��3] |
C | �����£�ʵ���ã�0.1mol��L��1Na2CO3��Һ��pHԼΪ11.6��0.1mol��L��1 NaHCO3��Һ��pHԼΪ9.7 | CO32 |
D | ��3mLϡ����ֱ����������Zn����Zn�۷�Ӧ��Zn�۲�����������ʿ� | Zn�۵Ļ����Դ���Zn�� |


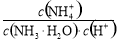 ��ֵ����
��ֵ���� ʣ��
ʣ�� �����H���������� HCO3����ǿ
�����H���������� HCO3����ǿ ��ϴ���Թ��ڱڵ�������Ag+2H��+NO3����Ag��+NO��+H2O
��ϴ���Թ��ڱڵ�������Ag+2H��+NO3����Ag��+NO��+H2O 2OH����H2����Cl2��
2OH����H2����Cl2�� ����ȫȼ�գ��������������Ĺ������ƹ�����ȫ��Ӧ����Ӧ����������ǡ��Ҳ������ng�������������������������������A��H2��CO�Ļ����B��C2H2O2C��C3H6O3D��C6H12O5
����ȫȼ�գ��������������Ĺ������ƹ�����ȫ��Ӧ����Ӧ����������ǡ��Ҳ������ng�������������������������������A��H2��CO�Ļ����B��C2H2O2C��C3H6O3D��C6H12O5