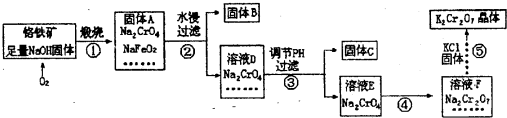
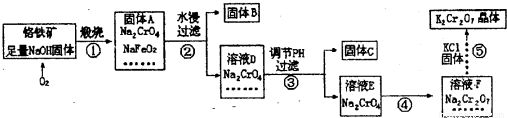
��Ŀ����
��֪�ظ����K2Cr2O7��Ũ�����ڳ����·�Ӧ�������������Իش��������⣺
��1������ɲ���ƽ���µĻ�ѧ����ʽ����δ֪��Ļ�ѧʽ�ͻ�ѧ���������룩
______K2Cr2O7+______HCl�T______KCl+______CrCl3+______Cl2��+______��
��2��Ũ������������Ӧ����ʾ����д��ţ�______
A��ֻ�л�ԭ�� B��ֻ�������� C��ֻ������ D���������������л�ԭ�� E�������������л�ԭ��
��3������Ӧ��ת��0.6mol ���ӣ��������Ļ�ԭ�������ʵ���Ϊ______ mol��������Cl2�������STP��Ϊ______L��
��4����֪ClO2�����壩���к�ǿ�������ԣ��ʳ���������������������Ч�ʣ��Ե�λ�������������õ����ӵ����ʵ�����С����ʾ�� Լ��Cl2��______��������2λС����
��1������ɲ���ƽ���µĻ�ѧ����ʽ����δ֪��Ļ�ѧʽ�ͻ�ѧ���������룩
______K2Cr2O7+______HCl�T______KCl+______CrCl3+______Cl2��+______��
��2��Ũ������������Ӧ����ʾ����д��ţ�______
A��ֻ�л�ԭ�� B��ֻ�������� C��ֻ������ D���������������л�ԭ�� E�������������л�ԭ��
��3������Ӧ��ת��0.6mol ���ӣ��������Ļ�ԭ�������ʵ���Ϊ______ mol��������Cl2�������STP��Ϊ______L��
��4����֪ClO2�����壩���к�ǿ�������ԣ��ʳ���������������������Ч�ʣ��Ե�λ�������������õ����ӵ����ʵ�����С����ʾ�� Լ��Cl2��______��������2λС����
��1��CrԪ�ػ��ϼ۱仯Ϊ��K2Cr2O7��CrCl3�����ϼ���+6��+3�ۣ�һ��ԭ�ӵ�3�����ӣ�����ԭ�ӵ�6�����ӣ�ClԪ�صĻ��ϼ۱仯Ϊ��HCl��Cl2�����ϼ���-1�ۡ�0�ۣ�һ��ԭ��ʧȥһ�����ӣ���������С������Ϊ6��K2Cr2O7�ļ�����Ϊ1������ԭ���� HCl�ļ�����Ϊ6���ٸ���ԭ���غ��ж��������ӵļ�����������Ԫ���غ㣬�������е�δ֪��Ϊˮ�����Ը÷���ʽΪ��K2Cr2O7+14HCl�T2KCl+2CrCl3+3Cl2��+7H2O��
�ʴ�Ϊ��1��14��2��2��3��7H2O
��2��HCl��ClԪ�صĻ��ϼۼ��б仯��Ҳ��δ�仯�ģ����ϼ۱仯���Ȼ����ǻ�ԭ�������ϼ�û�仯����������ã�
��ѡE��
��3��K2Cr2O7+6 HCl����ԭ����+8HCl�T2KCl+2CrCl3+3Cl2��+7H2O ת�Ƶ���
6mol 3��22.4L 6mol
0.6mol 6.72L 0.6mol
�ʴ�Ϊ��0.6mol�� 6.72L
��4��������������mg��ClO2��Cl-��5�����ӣ�Cl2��2Cl-��2�����ӣ�
����ClO2�õ��ĵ��ӵ����ʵ���Ϊ
��5=
mol��
Cl2�õ��ĵ��ӵ����ʵ���Ϊ
��2=
mol��
����
mol��
mol=2.63��
�ʴ�Ϊ2.63����
�ʴ�Ϊ��1��14��2��2��3��7H2O
��2��HCl��ClԪ�صĻ��ϼۼ��б仯��Ҳ��δ�仯�ģ����ϼ۱仯���Ȼ����ǻ�ԭ�������ϼ�û�仯����������ã�
��ѡE��
��3��K2Cr2O7+6 HCl����ԭ����+8HCl�T2KCl+2CrCl3+3Cl2��+7H2O ת�Ƶ���
6mol 3��22.4L 6mol
0.6mol 6.72L 0.6mol
�ʴ�Ϊ��0.6mol�� 6.72L
��4��������������mg��ClO2��Cl-��5�����ӣ�Cl2��2Cl-��2�����ӣ�
����ClO2�õ��ĵ��ӵ����ʵ���Ϊ
| mg |
| 67��5g/mol |
| m |
| 13��5 |
Cl2�õ��ĵ��ӵ����ʵ���Ϊ
| mg |
| 71g/mol |
| 2m |
| 71 |
����
| m |
| 13.5 |
| 2m |
| 71 |
�ʴ�Ϊ2.63����

��ϰ��ϵ�д�
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
�����Ŀ