��Ŀ����
��ҵ���ø�������Ҫ�ɷ�FeO?Cr2O3������ΪSiO2��Al2O3�ȣ������ظ���أ���ѧʽK2Cr2O�������������£�
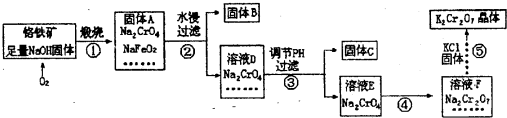
��1������A�У���Na2CrO4��NaFeO2��NaOH���______�ȣ�
��2������Aˮ��ʱ��NaFeO2�ᷢ��ǿ��ˮ���������B���仯ѧ��Ӧ����ʽΪ______��
��3������C�к���2�����ʣ�Ҫ�ȼ������������е�______��Ȼ����ܽ��й��˷��룮
A������������Һ B��ϡ��ˮ C��ϡ����
��4������ܼ�������õ�����ҺF�У���Na2Cr2O7�⣬�����е�������______��
��5�������������У���������ԭ��Ӧ�������ǵ�______����
��6����֪20��ʱK2Cr2O7������ҺŨ��Ϊ0.4mol?L-1��������м�������Ȼ��أ���ʹK+����Ũ�ȴﵽ4mol?L-1�������¶�20�棩������ҺF��Cr2O2-7���ӵ�Ũ�����Ϊ______��

��1������A�У���Na2CrO4��NaFeO2��NaOH���______�ȣ�
��2������Aˮ��ʱ��NaFeO2�ᷢ��ǿ��ˮ���������B���仯ѧ��Ӧ����ʽΪ______��
��3������C�к���2�����ʣ�Ҫ�ȼ������������е�______��Ȼ����ܽ��й��˷��룮
A������������Һ B��ϡ��ˮ C��ϡ����
��4������ܼ�������õ�����ҺF�У���Na2Cr2O7�⣬�����е�������______��
��5�������������У���������ԭ��Ӧ�������ǵ�______����
��6����֪20��ʱK2Cr2O7������ҺŨ��Ϊ0.4mol?L-1��������м�������Ȼ��أ���ʹK+����Ũ�ȴﵽ4mol?L-1�������¶�20�棩������ҺF��Cr2O2-7���ӵ�Ũ�����Ϊ______��
��1������Ŀ��Ϣ��֪�����������Ҫ�ɷ�FeO?Cr2O3������SiO2��Al2O3�����ʣ�SiO2��NaOH������Ӧ����Na2SiO3��Al2O3��NaOH������Ӧ������NaAlO2���ʻ�����Na2SiO3��NaAlO2��
�ʴ�Ϊ��Na2SiO3��NaAlO2��
��2��NaFeO2ˮ����������������������������Ӧ����ʽΪNaFeO2+2H2O�TNaOH+Fe��OH��3���ʴ�Ϊ��NaFeO2+2H2O�TNaOH+Fe��OH��3��
��3���ɹ������̿�֪������C�к���������������ᣬ����������Һǿ��ǿ���������ǿ��������ᣨHF���⣩��Ӧ����Ӧ�������ᣬ����������ת��Ϊƫ�����ƣ��ٹ��˷��룬
��ѡ��C��
��4���ɹ������̿�֪��Na2CrO4������������ת��ΪNa2Cr2O7������Ԫ���غ㻹���Ȼ������ɣ��ʴ�Ϊ��NaCl��
��5���ɹ������̿�֪���ڢٲ���Ӧ�У���Ԫ�ء���Ԫ�ء���Ԫ�ػ��ϼ۷�Ӧ�仯������������ԭ��Ӧ������������Ӧ��Ԫ�صĻ��ϼ�δ�仯��������������ԭ��Ӧ��
�ʴ�Ϊ���٣�
��6��20��ʱK2Cr2O7������ҺŨ��Ϊ0.4mol?L-1�����¶���c2��K+��?c��Cr2O2-7��=0.82?0.4=0.256������ʹK+����Ũ�ȴﵽ4mol?L-1�������¶�20�棩����ҺF��Cr2O2-7���ӵ�Ũ�����Ϊ
mol/L=0.016mol/L���ʴ�Ϊ��0.016mol/L��
�ʴ�Ϊ��Na2SiO3��NaAlO2��
��2��NaFeO2ˮ����������������������������Ӧ����ʽΪNaFeO2+2H2O�TNaOH+Fe��OH��3���ʴ�Ϊ��NaFeO2+2H2O�TNaOH+Fe��OH��3��
��3���ɹ������̿�֪������C�к���������������ᣬ����������Һǿ��ǿ���������ǿ��������ᣨHF���⣩��Ӧ����Ӧ�������ᣬ����������ת��Ϊƫ�����ƣ��ٹ��˷��룬
��ѡ��C��
��4���ɹ������̿�֪��Na2CrO4������������ת��ΪNa2Cr2O7������Ԫ���غ㻹���Ȼ������ɣ��ʴ�Ϊ��NaCl��
��5���ɹ������̿�֪���ڢٲ���Ӧ�У���Ԫ�ء���Ԫ�ء���Ԫ�ػ��ϼ۷�Ӧ�仯������������ԭ��Ӧ������������Ӧ��Ԫ�صĻ��ϼ�δ�仯��������������ԭ��Ӧ��
�ʴ�Ϊ���٣�
��6��20��ʱK2Cr2O7������ҺŨ��Ϊ0.4mol?L-1�����¶���c2��K+��?c��Cr2O2-7��=0.82?0.4=0.256������ʹK+����Ũ�ȴﵽ4mol?L-1�������¶�20�棩����ҺF��Cr2O2-7���ӵ�Ũ�����Ϊ
| 0.256 |
| 42 |

��ϰ��ϵ�д�
�����Ŀ



