��Ŀ����
ij����С����ע��������Ӧ������ͭ��ϡ���ᷴӦ��ʵ������˴�����ơ�����ʵ������ش��й����⣨�ٶ�ʵ���ڱ�״���½��У�������N2��O2�����֮��Ϊ4��1����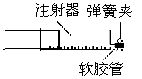
��1��ͼʾ��һ֧���Ϊ50mL��ע��������������Ͳ����������ã�����ע�����м���48mgͭƬ��Ȼ��γ���ͷ���ڰ���ͷ�����������ܡ�
��2���ȳ�ȡ����6mol/L��ϡHNO3��������������Һ���У�Ŀ����___________��
��3���ٳ�ȡ10mL6mol/L��ϡHNO3���ѵ��ɼм����������ϣ���Ӧ��ʼʱ������Ȼ���ӿ졣Ԥ����ע�����пɹ۲쵽��Щ����������д����
��4����Ӧ��Ϻ�ֹˮ�У���������������36.2mL�����кõ��ɼУ��۲쵽_______����ҡע�������£����ã��ֹ۲쵽_______��________���ظ����ϲ������Σ�����Ͳ�������NOΪֹ����Ͳ����Һ��c��NO��-��Ϊ_______��c��H+��Ϊ________������������������Ϊ______��
������
�����������
�����������Ƕ�ͭ�����ᷴӦʵ��Ĵ�����ƣ�Ҳ�Ƕ�˼ά�����Ĵ��¿��顣�ڻش�ʵ������ʱҪ����������ע��˼ά�������ԣ��ڽ��м���ʱҪץס���ʵ����Ĺ�ϵ��ע��˼ά������ԡ�
��2����Ŀ����Ϊ���ų�ע�����ڲ���������������
��3�����ȷ�����Ӧ�Ļ�ѧ����ʽ��������ʵ���龰���������ش𣺢�ͭƬ�����������ݳ�����Ͳ�ھۼ���ɫ���壻����Һ����ɫ��Ϊ��ɫ������ͭ�����ᷴӦ�����ʵ����ж��������֪��ͭƬȫ���ܽ⣻��ͨ������48mgͭƬ��ȫ��Ӧ����������ˮ��NO11.2mL֪�������Զ��Ƶ��̶�Լ21.2mL����
��4��NO������Ŀ�������Ϊ����ɫ��NO2������ɫ��NO2��ˮ��Ӧ�ֱ�Ϊ��ɫ����֪��4NO+3O2+2H2O=4HNO3���������������7mL��15mL������Լ��3mLO2����������36.2mL�������Ƶ�Լ29.2mL�����ӽ����NO��-Ũ���غ㣬��Ϊ6mol/L�����ܷ�Ӧ��2Cu+O2+4H+=2Cu2++2H2O�����H+��Ũ��Ϊ��10mL��6��10-3mol/L-0.048g/64g?mol-1��
 ��4���£�10��10-3L��=5.85mol/L��������������Ϊ0.048g/64g?mol-1��1/2��5��22400mL?mol-1=42mL��
��4���£�10��10-3L��=5.85mol/L��������������Ϊ0.048g/64g?mol-1��1/2��5��22400mL?mol-1=42mL�� 
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ij����С������ͼ��ʾװ�ý���ʵ�飨���Һ������������˵����ȷ���ǣ�������
ij����С������ͼ��ʾװ�ý���ʵ�飨���Һ������������˵����ȷ���ǣ�������

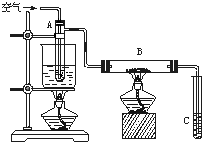 ij����С������ͼ��ʾ�����Ҵ��Ĵ�����ʵ��̽�����Թ�A��ʢ����ˮ�Ҵ���B��װ��CuO����ʯ���������壩���Թ�C�зŵ�������ˮ����ش��������⣺
ij����С������ͼ��ʾ�����Ҵ��Ĵ�����ʵ��̽�����Թ�A��ʢ����ˮ�Ҵ���B��װ��CuO����ʯ���������壩���Թ�C�зŵ�������ˮ����ش��������⣺