��Ŀ����
 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��������������ֲ�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��������������ֲ�ͬ����ش�
��1����A��B��C�о���ͬһ�ֳ�������Ԫ�أ���Ԫ����C������������ʽ���ڣ���A�к��еĽ���Ԫ�ص�����Ϊ______��
��2����AΪ��̬�ǽ������ʣ�A��Xͬ���ڣ����³�ѹ��CΪ��ɫ���壬B�����и�ԭ��������Ϊ8���ӽṹ����B�ĵ���ʽΪ______����C��ˮ���ҷ�Ӧ���������ֳ����ᣬ��Ӧ�Ļ�ѧ����ʽΪ��______��
��3����A��B��C����ɫ��Ӧ���ʻ�ɫ��ˮ��Һ��Ϊ���ԣ�
��A�������еĻ�ѧ����______��______�ڽ�4.48L����״���£�Xͨ��100mL3mol/L A��ˮ��Һ����Һ������Ũ���ɴ�С��˳��Ϊ______��
�⣺��1����A��B��C�о���ͬһ�ֳ�������Ԫ�أ���Ԫ����C������������ʽ���ڣ���ת����ϵ��֪��A���������ӣ�BΪ����������C����ƫ�������xΪ�������ƣ�A��B��C�к��е�ͬһ�ֳ�������Ԫ��ΪAl��
�ʴ�Ϊ��Al��
��2����AΪ��̬�ǽ������ʣ�A��XͬΪ��������Ԫ�أ����³�ѹ��CΪ��ɫ���壬B�����и�ԭ��������Ϊ8e-�ṹ����ת����ϵ��֪��AΪ�ף�BΪ���Ȼ��ף�CΪ���Ȼ��ף�xΪ������
��BΪ���Ȼ��ף���ԭ�Ӷ���8�����ȶ��ṹ�����Ȼ���B�ĵ���ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��CΪ���Ȼ��ף���ˮ���ҷ�Ӧ��������������ᣬ��Ӧ�Ļ�ѧ����ʽΪPCl5+4H2O=H3PO4+5HC1��
�ʴ�Ϊ��PCl5+4H2O=H3PO4+5HC1��
��3����A��B��C����ɫ��Ӧ���ʻ�ɫ��������Ԫ�أ�ˮ��Һ��Ϊ���ԣ���ת����ϵ����AΪ�������ƣ�BΪ̼���ƣ�CΪ̼�����ƣ�xΪ������̼��
��AΪ�������ƣ��������ӻ����������������������֮���γ����Ӽ�����������������ԭ������ԭ��֮���γ�1�Թ��õ��Ӷԣ�Ϊ���ۼ�������A�������еĻ�ѧ���� ���Ӽ������ۼ���
�ʴ�Ϊ�����Ӽ������ۼ���
��4.48L����״���£�CO2�����ʵ���Ϊ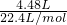 =0.2mol��100mL3mol/L NaOH��ˮ��Һ��n��NaOH��=0.1L��3mol/L=0.3mol��n��CO2����n��NaOH��=0.2mol��0.3mol=2��3=1��1.5������1��1��1��2֮�䣬�ʷ�Ӧ����̼������̼�����ƣ���̼������̼�����Ƶ����ʵ����ֱ�Ϊamol��bmol�����������غ���2a+b=0.3����̼Ԫ���غ���a+b=0.2���������̣����a=0.1��b=0.1��̼�����̼�����ˮ�⣬��Һ�ʼ���c��OH-����c��H+����̼�����ˮ��̶ȱ�̼������Ĵ�c��HCO3-����c��CO32-����ˮ��̶Ⱥ�С��c��CO32-��ԭ����c��OH-������Һ���������Ѷ���ʣ�c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
=0.2mol��100mL3mol/L NaOH��ˮ��Һ��n��NaOH��=0.1L��3mol/L=0.3mol��n��CO2����n��NaOH��=0.2mol��0.3mol=2��3=1��1.5������1��1��1��2֮�䣬�ʷ�Ӧ����̼������̼�����ƣ���̼������̼�����Ƶ����ʵ����ֱ�Ϊamol��bmol�����������غ���2a+b=0.3����̼Ԫ���غ���a+b=0.2���������̣����a=0.1��b=0.1��̼�����̼�����ˮ�⣬��Һ�ʼ���c��OH-����c��H+����̼�����ˮ��̶ȱ�̼������Ĵ�c��HCO3-����c��CO32-����ˮ��̶Ⱥ�С��c��CO32-��ԭ����c��OH-������Һ���������Ѷ���ʣ�c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
������A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�
��1����A��B��C�о���ͬһ�ֳ�������Ԫ�أ���Ԫ����C������������ʽ���ڣ���ת����ϵ������CΪAlO2-��A���������ӣ�BΪ����������C����ƫ�������xΪ�������ƣ�����ת����ϵ��
��2����AΪ��̬�ǽ������ʣ�A��XͬΪ��������Ԫ�أ����³�ѹ��CΪ��ɫ���壬B�����и�ԭ��������Ϊ8e-�ṹ����ת����ϵ��֪��AΪ�ף�BΪ���Ȼ��ף�CΪ���Ȼ��ף�xΪ������
��3����A��B��C����ɫ��Ӧ���ʻ�ɫ��������Ԫ�أ�ˮ��Һ��Ϊ���ԣ���ת����ϵ��AΪ�������ƣ�BΪ̼���ƣ�CΪ̼�����ƣ�xΪ������̼��
����������Ԫ�ػ������ƶϣ��Ѷ��еȣ��ؼ����ճ���Ԫ�ػ���������ʣ�����ת����ϵѡ����ʵ����ʽ��н��
�ʴ�Ϊ��Al��
��2����AΪ��̬�ǽ������ʣ�A��XͬΪ��������Ԫ�أ����³�ѹ��CΪ��ɫ���壬B�����и�ԭ��������Ϊ8e-�ṹ����ת����ϵ��֪��AΪ�ף�BΪ���Ȼ��ף�CΪ���Ȼ��ף�xΪ������
��BΪ���Ȼ��ף���ԭ�Ӷ���8�����ȶ��ṹ�����Ȼ���B�ĵ���ʽΪ
 ��
���ʴ�Ϊ��
 ��
����CΪ���Ȼ��ף���ˮ���ҷ�Ӧ��������������ᣬ��Ӧ�Ļ�ѧ����ʽΪPCl5+4H2O=H3PO4+5HC1��
�ʴ�Ϊ��PCl5+4H2O=H3PO4+5HC1��
��3����A��B��C����ɫ��Ӧ���ʻ�ɫ��������Ԫ�أ�ˮ��Һ��Ϊ���ԣ���ת����ϵ����AΪ�������ƣ�BΪ̼���ƣ�CΪ̼�����ƣ�xΪ������̼��
��AΪ�������ƣ��������ӻ����������������������֮���γ����Ӽ�����������������ԭ������ԭ��֮���γ�1�Թ��õ��Ӷԣ�Ϊ���ۼ�������A�������еĻ�ѧ���� ���Ӽ������ۼ���
�ʴ�Ϊ�����Ӽ������ۼ���
��4.48L����״���£�CO2�����ʵ���Ϊ
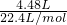 =0.2mol��100mL3mol/L NaOH��ˮ��Һ��n��NaOH��=0.1L��3mol/L=0.3mol��n��CO2����n��NaOH��=0.2mol��0.3mol=2��3=1��1.5������1��1��1��2֮�䣬�ʷ�Ӧ����̼������̼�����ƣ���̼������̼�����Ƶ����ʵ����ֱ�Ϊamol��bmol�����������غ���2a+b=0.3����̼Ԫ���غ���a+b=0.2���������̣����a=0.1��b=0.1��̼�����̼�����ˮ�⣬��Һ�ʼ���c��OH-����c��H+����̼�����ˮ��̶ȱ�̼������Ĵ�c��HCO3-����c��CO32-����ˮ��̶Ⱥ�С��c��CO32-��ԭ����c��OH-������Һ���������Ѷ���ʣ�c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
=0.2mol��100mL3mol/L NaOH��ˮ��Һ��n��NaOH��=0.1L��3mol/L=0.3mol��n��CO2����n��NaOH��=0.2mol��0.3mol=2��3=1��1.5������1��1��1��2֮�䣬�ʷ�Ӧ����̼������̼�����ƣ���̼������̼�����Ƶ����ʵ����ֱ�Ϊamol��bmol�����������غ���2a+b=0.3����̼Ԫ���غ���a+b=0.2���������̣����a=0.1��b=0.1��̼�����̼�����ˮ�⣬��Һ�ʼ���c��OH-����c��H+����̼�����ˮ��̶ȱ�̼������Ĵ�c��HCO3-����c��CO32-����ˮ��̶Ⱥ�С��c��CO32-��ԭ����c��OH-������Һ���������Ѷ���ʣ�c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+�����ʴ�Ϊ��c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
������A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�
��1����A��B��C�о���ͬһ�ֳ�������Ԫ�أ���Ԫ����C������������ʽ���ڣ���ת����ϵ������CΪAlO2-��A���������ӣ�BΪ����������C����ƫ�������xΪ�������ƣ�����ת����ϵ��
��2����AΪ��̬�ǽ������ʣ�A��XͬΪ��������Ԫ�أ����³�ѹ��CΪ��ɫ���壬B�����и�ԭ��������Ϊ8e-�ṹ����ת����ϵ��֪��AΪ�ף�BΪ���Ȼ��ף�CΪ���Ȼ��ף�xΪ������
��3����A��B��C����ɫ��Ӧ���ʻ�ɫ��������Ԫ�أ�ˮ��Һ��Ϊ���ԣ���ת����ϵ��AΪ�������ƣ�BΪ̼���ƣ�CΪ̼�����ƣ�xΪ������̼��
����������Ԫ�ػ������ƶϣ��Ѷ��еȣ��ؼ����ճ���Ԫ�ػ���������ʣ�����ת����ϵѡ����ʵ����ʽ��н��

��ϰ��ϵ�д�
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ
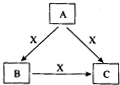 A��B��C��X����ѧ��ѧ�������ʣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش����⣺
A��B��C��X����ѧ��ѧ�������ʣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش����⣺ A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�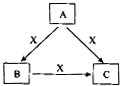 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ��������������ֲ�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ��������������ֲ�ͬ����ش�
 A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺ A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺