��Ŀ����
 ��һ����Ҫ�Ļ���ԭ�ϡ�ij�о���ѧϰС�������������þ��ʯ(��ˣ�ɷ���
��һ����Ҫ�Ļ���ԭ�ϡ�ij�о���ѧϰС�������������þ��ʯ(��ˣ�ɷ��� ��������
�������� ���ʣ���ȡ
���ʣ���ȡ ��ʵ�飬��������
��ʵ�飬��������
(1) �ڢٲ���ĥ��Ŀ����______________������Һ��Ҫ��______________��______________�����ʡ�
(2) �ڢڲ���Ӧ�����ӷ���ʽΪ____________________________��
(3) �ڢ۲�Ũ���ᾧ��Ҫ����_______��ϴ�ӡ�����Ȳ���ſɵõ� ��ϴ�ӳ����Ļ���������___________________________________��
��ϴ�ӳ����Ļ���������___________________________________��
(4) ���Ƶ� ����Ϊ82.00�ˣ����MnO2����Ϊ1.74�ˣ��Ҳ�õڢٲ���������Ϊ4. 70�ˣ����Ը���������Һ�е��ܽ���ʧ���ɼ������þ��ʯ��
����Ϊ82.00�ˣ����MnO2����Ϊ1.74�ˣ��Ҳ�õڢٲ���������Ϊ4. 70�ˣ����Ը���������Һ�е��ܽ���ʧ���ɼ������þ��ʯ�� ����������Ϊ_______��
����������Ϊ_______��
(2) �ڢڲ���Ӧ�����ӷ���ʽΪ____________________________��
(3) �ڢ۲�Ũ���ᾧ��Ҫ����_______��ϴ�ӡ�����Ȳ���ſɵõ�
 ��ϴ�ӳ����Ļ���������___________________________________��
��ϴ�ӳ����Ļ���������___________________________________�� (4) ���Ƶ�
 ����Ϊ82.00�ˣ����MnO2����Ϊ1.74�ˣ��Ҳ�õڢٲ���������Ϊ4. 70�ˣ����Ը���������Һ�е��ܽ���ʧ���ɼ������þ��ʯ��
����Ϊ82.00�ˣ����MnO2����Ϊ1.74�ˣ��Ҳ�õڢٲ���������Ϊ4. 70�ˣ����Ը���������Һ�е��ܽ���ʧ���ɼ������þ��ʯ�� ����������Ϊ_______��
����������Ϊ_______�� (1)ʹ�ܽ��ֲ��ӿ��ܽ����ʣ�MgSO4��MnSO4
(2)Mn2++ClO-+H2O=MnO2��+Cl-+2H+
(3)���ˣ����������ڹ������У�������ˮ�����պý�û��������ˮ�������ظ�2-3��
(4)80%
(2)Mn2++ClO-+H2O=MnO2��+Cl-+2H+
(3)���ˣ����������ڹ������У�������ˮ�����պý�û��������ˮ�������ظ�2-3��
(4)80%

��ϰ��ϵ�д�
�����Ŀ
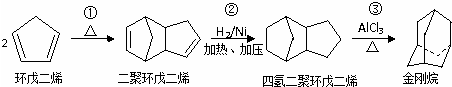
 ����һ����Ҫ�Ļ���ԭ�ϣ���ϳ�·�����£�
����һ����Ҫ�Ļ���ԭ�ϣ���ϳ�·�����£�
 ��Ӧ�Ļ�ѧ����ʽ��
��Ӧ�Ļ�ѧ����ʽ��
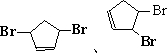

 ����д��A���п��ܵĽṹ��ʽ
����д��A���п��ܵĽṹ��ʽ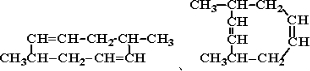
 ��̬��A�ڱ���µ��ܶ�Ϊ1.25g/L��A��һ����Ҫ�Ļ���ԭ�ϣ����IJ���ͨ����������һ������ʯ�ͻ���ˮƽ��B��D���������г������л��D�ܸ�̼��������Һ��Ӧ��E����ζ������֮���ת����ϵ��ͼ��ʾ��
��̬��A�ڱ���µ��ܶ�Ϊ1.25g/L��A��һ����Ҫ�Ļ���ԭ�ϣ����IJ���ͨ����������һ������ʯ�ͻ���ˮƽ��B��D���������г������л��D�ܸ�̼��������Һ��Ӧ��E����ζ������֮���ת����ϵ��ͼ��ʾ��


