��Ŀ����
�мס������������������ݻ��̶����������ݻ��ɱ䡣һ���¶��£��ڼ�����2 molN2��3 mol H2��N2(g)+3H2(g)(1)��ͬ�¶��£������м���4 mol N2��6 mol H2,���ҵ�ѹǿʼ�����ѹǿ��ȣ����з�Ӧ��ƽ��ʱ������NH3�����ʵ���Ϊ________mol(�����и�����ѡ��ֻ���������ͬ)�����ҵ��ݻ�����ݻ�ʼ����ȣ����з�Ӧ��ƽ��ʱ������NH3�����ʵ���Ϊ________mol��
A.С��m B.����m C.��m��2m֮��
D.����2m E.����2m
(2)��ͬ�¶��£������ҵ��ݻ�Ϊ��һ�룬������1 mol NH3��Ҫʹ���з�Ӧ��ƽ��ʱ�������ʵ���������������������д�ƽ��ʱ��ͬ������ʼӦ����______molN2��______mol H2��
(1)D E (2)0.5 0

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
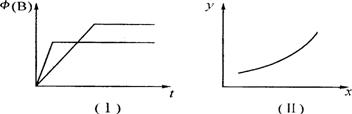
 B��g�� + 2C��g�� ��H= m kJ/mol������������ͼ���й�������ȷ����
B��g�� + 2C��g�� ��H= m kJ/mol������������ͼ���й�������ȷ����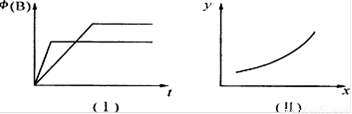
 xC��g���ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2��
xC��g���ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2��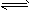 xC��g���ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2��
xC��g���ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2��