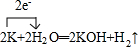��Ŀ����
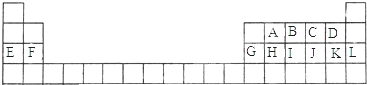
����4�֣���Ԫ�ط��Ż�ѧʽ�ش�ԭ������11��18��Ԫ�ص��й����⣺
��1����ϡ�������⣬ԭ�Ӱ뾶����Ԫ����________________��
��2������������Ӧˮ��������Ե�Ԫ����________________��
��3����������������Ӧˮ����������ǿ������________________��
��4�����γ���̬�⻯�����⻯�����ȶ���Ԫ����________________��
����4�֣���MgCl2��AlCl3�Ļ����Һ�У���μ���NaOH��Һֱ�����������ⶨ������NaOH�����ʵ��������ó��������ʵ����Ĺ�ϵ����ͼ��ʾ������Һ��n(Mg2+):n(Al3+)= ��AB�η��������ӷ�ӦΪ ��

���𰸡�
��ÿ��1�֣�(1)Na ��2��Al ��3��HClO4 (4)Cl
��(ÿ��2��)1��1 ��A1(OH)3 + OH�� = [A1(OH)4] ��
��������

��ϰ��ϵ�д�
 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ