��Ŀ����
��1�����ࡢ��֬������������������______�����������ԭ������������������Ҫ������������______�Ǻ�������ߵ�Ӫ�����ʣ������ܽ��ԵIJ�ͬ��ά����B������______����ά���أ�ά����D ����______����ά���أ�ijҩƷ��ǩ�����С�OTC�����ţ�����ʾ______��
��2��ҩ�����Ԥ������Ϻ����Ƽ������ܶѧ�Լ���������ɱ����Ч������ѧ��ѧ��Χ�ڣ�����Ϊҩ��Ļ�ѧ�Լ��ܶ࣬�������������ҡ�̼�����Ʒۼ��ȣ�д������������̼�����Ʒֱ���θ�����Ҫ�ɷ����õ����ӷ���ʽ��______��______����˿���������______��
��2��ҩ�����Ԥ������Ϻ����Ƽ������ܶѧ�Լ���������ɱ����Ч������ѧ��ѧ��Χ�ڣ�����Ϊҩ��Ļ�ѧ�Լ��ܶ࣬�������������ҡ�̼�����Ʒۼ��ȣ�д������������̼�����Ʒֱ���θ�����Ҫ�ɷ����õ����ӷ���ʽ��______��______����˿���������______��
��1�����ࡢ��֬�����������������з�����������ͬ���������ࡢ��֬���������ͷ�������ߵ�����֬��ά����B������ˮ����ά���أ�ά����D ����֬����ά���أ�ijҩƷ��ǩ�����С�OTC�����ţ�����ʾ�Ǵ���ҩ����R�����ţ�����ʾ����ҩ���ʴ�Ϊ����������֬��ˮ��֬���Ǵ���ҩ�
��2��θ�����Ҫ�ɷ�Ϊ���ᣬθ��������������Ӧ�����Ȼ�����ˮ����Ӧ�����ӷ���ʽΪAl��OH��3+3H+=Al3++3H2O��
θ����̼�����Ʒ�Ӧ�����Ȼ��ƺͶ�����̼���壬��Ӧ�����ӷ���ʽΪHCO3-+H+=CO2��+H2O������������̼�����ƿ���������θ����࣬�ʴ�Ϊ��Al��OH��3+3H+=Al3++3H2O��HCO3-+H+=CO2��+H2O��θ����࣮
��2��θ�����Ҫ�ɷ�Ϊ���ᣬθ��������������Ӧ�����Ȼ�����ˮ����Ӧ�����ӷ���ʽΪAl��OH��3+3H+=Al3++3H2O��
θ����̼�����Ʒ�Ӧ�����Ȼ��ƺͶ�����̼���壬��Ӧ�����ӷ���ʽΪHCO3-+H+=CO2��+H2O������������̼�����ƿ���������θ����࣬�ʴ�Ϊ��Al��OH��3+3H+=Al3++3H2O��HCO3-+H+=CO2��+H2O��θ����࣮

��ϰ��ϵ�д�
�����Ŀ
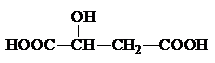 ���÷����й����ŵ�����Ϊ
���÷����й����ŵ�����Ϊ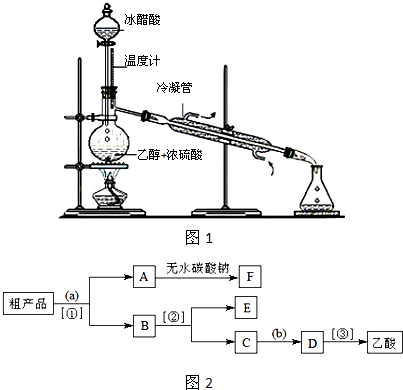
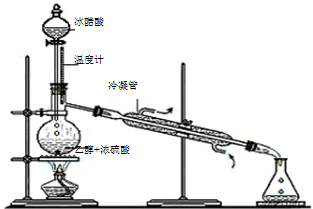 �л���ѧ֪ʶ��������Ӧ�ù㷺��
�л���ѧ֪ʶ��������Ӧ�ù㷺��