��Ŀ����
��ѧ����Ϊ��������һ�ָ�Ч������Ⱦ��������Դ����20����������������Ϊδ���Ķ���ȼ������Դ���о������Ѹ�ٷ�չ��
��1��Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������пɹ������ֽϾ�������Դ�ɳ������õ��������ķ�����
A�����ˮ B��п��ϡ���ᷴӦ C����⺣ˮ D����ʯ�͡���Ȼ��Ϊԭ��
��2��������Ϊ��Դؽ���������һ������������ϵĿ������о�����ijЩ���ɽ����磨La�����٣�Pd��������ԭ���γ��⻯���ԭ������ڽ��������ڵļ�϶֮�䣬����ɲ��̶���ͨ���Ƿǻ�ѧ�����ģ�������⻯��Ļ�ѧʽ�ɱ�ʾΪLaH2.76����֪�ٷ۵��ܶ�Ϊ10.64g/cm3�����ԭ������Ϊ106.4��1������ٷ۴�Լ��������״����896��������������ٵ��⻯��Ļ�ѧʽΪ
��3����������ȼ�ױ������˰�ȫ��Ϊ��Ҫ����֪������ȼ����Ϊ285.8kJ?mol-1��
д������ȫȼ�յ��Ȼ�ѧ����ʽ�� ��
��1��Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������пɹ������ֽϾ�������Դ�ɳ������õ��������ķ�����
A�����ˮ B��п��ϡ���ᷴӦ C����⺣ˮ D����ʯ�͡���Ȼ��Ϊԭ��
��2��������Ϊ��Դؽ���������һ������������ϵĿ������о�����ijЩ���ɽ����磨La�����٣�Pd��������ԭ���γ��⻯���ԭ������ڽ��������ڵļ�϶֮�䣬����ɲ��̶���ͨ���Ƿǻ�ѧ�����ģ�������⻯��Ļ�ѧʽ�ɱ�ʾΪLaH2.76����֪�ٷ۵��ܶ�Ϊ10.64g/cm3�����ԭ������Ϊ106.4��1������ٷ۴�Լ��������״����896��������������ٵ��⻯��Ļ�ѧʽΪ
��3����������ȼ�ױ������˰�ȫ��Ϊ��Ҫ����֪������ȼ����Ϊ285.8kJ?mol-1��
д������ȫȼ�յ��Ȼ�ѧ����ʽ��
��������1�������Ͼ�������Դ�ɳ������õ�����������Ҫ����Դ���ķ�����������
��2����1���Ϊ1cm3������ٷ۵����������ʵ������ٸ���1������ٷ۴�Լ��������״����896�����������������������ʵ�������������ԭ�ӵ����ʵ�����д����ѧʽ��
��3��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ų�������������ȼ������д�Ȼ�ѧ����ʽ��
��2����1���Ϊ1cm3������ٷ۵����������ʵ������ٸ���1������ٷ۴�Լ��������״����896�����������������������ʵ�������������ԭ�ӵ����ʵ�����д����ѧʽ��
��3��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ų�������������ȼ������д�Ȼ�ѧ����ʽ��
����⣺��1��A�����ˮ��Ҫ�����ĵ��ܣ����������۵�Ҫ��A����
B��п��ϡ���ᷴӦ�����Ĵ�����п�����ᣬ���������۵�Ҫ��B����
C����⺣ˮ���ɳ�����ù��ܣ����۶��ֵ�̼������Ҫ��C��ȷ��
D��ʯ�͡���Ȼ��Ϊ����������Դ����������Դ�ɳ������õ�Ҫ��D����
�ʴ�Ϊ��C��
��2����1���Ϊ1cm3����1cm3���ٷ۵�����m=��V=10.64g/cm3��1cm3=10.64g���ٵ����ʵ���n��Pd��=
=
=0.1mol��
��1����ٷ�Լ��������״����896�����������֪���������������Ϊ896cm3=0.896L�����������ʵ���n��H2��=
=
=0.04mol��
��������������Ӧ�����ʵ���֮��Ϊ
���������Pd��H֮��Ϊ
=
�������ٵ��⻯�ﻯѧʽΪPdH0.8��
�ʴ�Ϊ��PdH0.8��
��3��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ų�����������֪������ȼ����Ϊ285.8kJ?mol-1����1mol������ȫȼ������Һ̬ˮ�ų�285.8kJ���������������Ȼ�ѧ����ʽΪ��H2��g��+
O2��g���TH2O��l����H=-285.8 kJ/mol��
�ʴ�Ϊ��H2��g��+
O2��g���TH2O��l����H=-285.8 kJ/mol��
B��п��ϡ���ᷴӦ�����Ĵ�����п�����ᣬ���������۵�Ҫ��B����
C����⺣ˮ���ɳ�����ù��ܣ����۶��ֵ�̼������Ҫ��C��ȷ��
D��ʯ�͡���Ȼ��Ϊ����������Դ����������Դ�ɳ������õ�Ҫ��D����
�ʴ�Ϊ��C��
��2����1���Ϊ1cm3����1cm3���ٷ۵�����m=��V=10.64g/cm3��1cm3=10.64g���ٵ����ʵ���n��Pd��=
| m |
| M |
| 10.64g |
| 106.4g/mol |
��1����ٷ�Լ��������״����896�����������֪���������������Ϊ896cm3=0.896L�����������ʵ���n��H2��=
| V |
| V m |
| 0.896L |
| 22.4L/mol |
��������������Ӧ�����ʵ���֮��Ϊ
| 0.1 |
| 0.04 |
| 0.1 |
| 0.04��2 |
| 1 |
| 0.8 |
�ʴ�Ϊ��PdH0.8��
��3��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ų�����������֪������ȼ����Ϊ285.8kJ?mol-1����1mol������ȫȼ������Һ̬ˮ�ų�285.8kJ���������������Ȼ�ѧ����ʽΪ��H2��g��+
| 1 |
| 2 |
�ʴ�Ϊ��H2��g��+
| 1 |
| 2 |
���������⿼������Դ���⡢��ѧʽ�����㡢�Ȼ�ѧ����ʽ����д������֪ʶӦ�÷���Ŀ��飬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
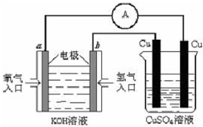
 ��ѧ����Ϊ��������һ�ָ�Ч������Ⱦ��������Դ��Ϊ����CO�Դ�������Ⱦ��ij�о���ѧϰС�����о�CO��H2O��Ӧת��Ϊ��ɫ��ԴH2��
��ѧ����Ϊ��������һ�ָ�Ч������Ⱦ��������Դ��Ϊ����CO�Դ�������Ⱦ��ij�о���ѧϰС�����о�CO��H2O��Ӧת��Ϊ��ɫ��ԴH2��
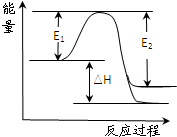
 CH3OH(g)����H ����90.8 kJ��mol��1
CH3OH(g)����H ����90.8 kJ��mol��1