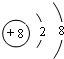��Ŀ����
����Ԫ��A��B��C��D��E��ԭ�����������������ǵ�ԭ�Ӻ�����Ӳ���֮��Ϊ10��A��Eͬ���塣����Ȼ���У�BԪ���γɵĻ�����������ࡣC��D��Ԫ��ԭ�ӵ�����������֮��Ϊ11����ش��������⣺(1)C��D�γɵij����������Ǵ�������Ⱦ��֮һ����Σ����_______________(�о�һ��)
(2)����������X��Y����A��B��D��E����Ԫ����ɣ�X���������д������X��Һ������Ba(OH)2��Һ��Ӧ�����ӷ���ʽ_______________��Y�Ļ�ѧʽΪB
(3)��֪C2(g)+D2(g)![]() 2CD(g) ��H=180.5 kJ��mol-1
2CD(g) ��H=180.5 kJ��mol-1
4CA3(g)+5D2(g)![]() 4CD(g)+
4CD(g)+
![]()
д����ҵ��C2��A2��Ӧ���Ȼ�ѧ����ʽ______________________________��
(4)��һ�������£�A2��C2�����¶Ⱥ㶨�ݻ�Ϊ

�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ______________________________��
�ڵ�25 min��ƽ��ı��������_______________�����´ﵽƽ����ܷ�����CA3���������_______________(��ܡ���)��
(1)������꣬�γɹ⻯ѧ����(��дһ��)
(2)![]() +Ba2++OH-
+Ba2++OH-![]() BaCO3��+H2O
BaCO3��+H2O
1.0��10-6mol��L-1
(3)N2(g)+3H2(g)![]() 2NH3(g) ��H=-92.4kJ��mol-1
2NH3(g) ��H=-92.4kJ��mol-1
(4)�� K=![]() �ڷ����NH3 ��
�ڷ����NH3 ��
������A![]() E����ΪH��C��N��O��Na��
E����ΪH��C��N��O��Na��
(1)�γ����꣬�γɹ⻯ѧ������
(2)XΪNaHCO3��YΪCH3COONa
![]() +Ba2++OH-
+Ba2++OH-![]() BaCO3��+H2O
BaCO3��+H2O
�������غ��c(H+)+c(CH3COOH)=c(OH-)=10-6 mol��L-1
(3)N2(g)+3H2(g)![]() 2NH3(g) ��H=-92.4 kJ��mol-1
2NH3(g) ��H=-92.4 kJ��mol-1
(4)��K=![]()
�ڵ�25 min��NH3ͻȻ���٣�N2��H2δ�ı䣬���ƽ��ı�������Ƿ����NH3�����´�ƽ����ܼ����NH3�����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�