��Ŀ����
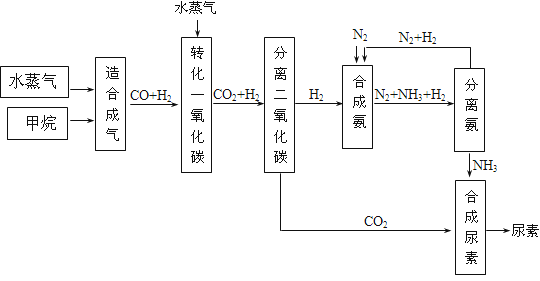
29.�Ĵ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ��ͼ��ijЩת�����輰������δ�г�����
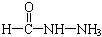
����д���пհף�
��1����֪0.5 mol������0.5 molˮ������t �桢p kPaʱ����ȫ��Ӧ����һ����̼���������ϳ�������������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ� ��
��2���ںϳɰ���ʵ�����������У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪���������������û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ� ��
��3��������ϳɰ�����ת����Ϊ75%ʱ����5.60��107 L����Ϊԭ���ܹ��ϳɣ� L������������������ڱ�״���²ⶨ��
��4����֪���صĽṹ��ʽΪ![]() ��д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
��д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
�� ���� ��
��1��CH4��g��+H2O��g��![]() CO��g��+3H2��g������H=2a kJ/mol
CO��g��+3H2��g������H=2a kJ/mol
��2����������������Ũ������������Ӧ���ʣ���С������Ũ�ȣ���������������Ũ�Ⱦ�������ƽ��������Ӧ�����ƶ�
��3��1.12��108
��4����![]() ��NH4N=C=O
��NH4N=C=O
���������ݺϳ�·��ͼ�����·�Ӧ��CH4+H2O=CO+3H2��CO+H2O=CO2+H2��N2+3H2 ![]() 2NH3�������õ���ϵ��CH4��4H2��
2NH3�������õ���ϵ��CH4��4H2��![]() NH3�������ܺϳɰ�����
NH3�������ܺϳɰ�����![]() ��
��

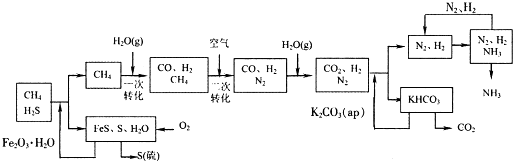

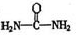 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����
����
��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����
����
��