��Ŀ����
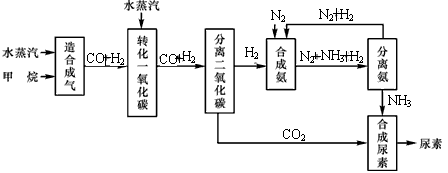
�Ĵ��зḻ����Ȼ����Դ��ij����������Ȼ��Ϊԭ�Ϻϳɰ��Ĺ�������ʾ��ͼ���£�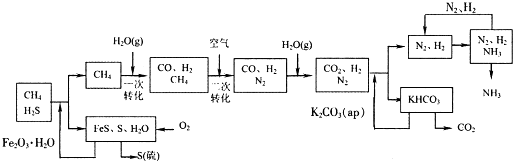
�����������̣����������գ�
��1����������������ѭ����һ��K2CO3��aq��ѭ��������N2��H2ѭ����������ѭ���б�ѭ����������
��2����������У�����nmol Fe2O3?H2Oת����������S�����ʵ���Ϊ
��3��ͼ��CH4�ĵڶ���ת�������з�����Ӧ�Ļ�ѧ����ʽΪ
��4���ںϳɰ���ʵ�����������У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪���������������û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ȡ��Щ��ʩ�����ɣ�
��5�����ù���NaOH��Һ������Ȼ���е����⣬��ʯī���缫������պ�������Һ�ɻ����������ܷ�Ӧ����ʽ������������������ԭ��Ϊ
��6������ҵ��������a g�����������Ŀ���Ϊԭ�ϣ������ǿ�����N2�ķ�Ӧ������ȵ���ȡNH4NO3������һϵ��ת��������Ӧ��Ļ�����м���bgˮ���õ��ܶ�Ϊd g/mL��NH4NO3��Һ�������ϸ���Һ�����ʵ����ʵ���Ũ�ȿ��ܵ����ֵΪ
��������1��������������ͼ�����漰�����ʵ���Դ��ȥ��ش�
��2������H2S����Fe2O3?H2O��Ӧ��3H2S+Fe2O3?H2O=2FeS+S+4H2O�����㣻
��3�����ݹ�������ʾ��ͼ��֪CH4�ĵ�һ��ת��������������CO��H2��
��4����������������ɼ���������Ũ�ȣ���ѧƽ�������ƶ������䵪��������������������Ũ�ȣ���������Ӧ���ʣ�
��5������NaOH��Һ������Ȼ���е���������Na2S�����ʱ���������ӷŵ����ɵ��������������ӷŵ�����������
��6���������⣬���̶ȵ���������泥���Ϸ���ʽ������������������ʵ������������������淋����ʵ���Ũ�ȣ�
��2������H2S����Fe2O3?H2O��Ӧ��3H2S+Fe2O3?H2O=2FeS+S+4H2O�����㣻
��3�����ݹ�������ʾ��ͼ��֪CH4�ĵ�һ��ת��������������CO��H2��
��4����������������ɼ���������Ũ�ȣ���ѧƽ�������ƶ������䵪��������������������Ũ�ȣ���������Ӧ���ʣ�
��5������NaOH��Һ������Ȼ���е���������Na2S�����ʱ���������ӷŵ����ɵ��������������ӷŵ�����������
��6���������⣬���̶ȵ���������泥���Ϸ���ʽ������������������ʵ������������������淋����ʵ���Ũ�ȣ�
����⣺��1���ɹ�������ʾ��ͼ��֪������ѭ���б�ѭ��������Fe2O3?H2Oѭ�����ʴ�Ϊ��Fe2O3?H2O��
��2����H2S����Fe2O3?H2O��Ӧ��3H2S+Fe2O3?H2O=2FeS+S+4H2O��֪����nmolFe2O3?H2Oת��������S�����ʵ���Ϊnmol���ʴ�Ϊ��n��
��3��CH4�ĵ�һ��ת��������������CO��H2������ʽΪCH4+H2O?CO+3H2���ʴ�Ϊ��CH4+H2O?CO+3H2��
��4���ںϳɰ���ʵ�����������У���ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪�������������������͵�����Ũ������������Ӧ���ʣ����������͵���Ũ�ȣ����ٰ���Ũ��������ƽ��������У�
�ʴ�Ϊ����������������Ũ������������Ӧ���ʣ���С������Ũ�ȣ���������������Ũ�Ⱦ�������ƽ��������Ӧ�����ƶ���
��5��NaOH��Һ������Ȼ���е���������Na2S�����ʱ���������ӷŵ����ɵ��������������ӷŵ���������������ܷ�Ӧ����ʽΪ��Na2S+2H2O
2NaOH+S+H2����
�ʴ�Ϊ��Na2S+2H2O
2NaOH+S+H2����
��6����
mol NH3����x mol�����������ᣬ���ࣨ
-x�� mol NH3�����ɵ�����ǡ����ȫ��Ӧ����NH4NO3��
��4NH3+5O2�T4NO+6H2O��4NO+3O2+2H2O�T4HNO3��
NH3��NO��HNO3 NH3 +HNO3 �TNH4NO3
x mol x mol x mol ��
-x��mol x mol
�����⣬���̶���ȡNH4NO3����Ӧ�����㣨
-x��=x��
��� x=
��
���ԣ���������ʵ���Ũ���ǣ�c��NH4NO3���T
=
=
=
mol/Lmol?L-1��
�ʴ�Ϊ��
��
��2����H2S����Fe2O3?H2O��Ӧ��3H2S+Fe2O3?H2O=2FeS+S+4H2O��֪����nmolFe2O3?H2Oת��������S�����ʵ���Ϊnmol���ʴ�Ϊ��n��
��3��CH4�ĵ�һ��ת��������������CO��H2������ʽΪCH4+H2O?CO+3H2���ʴ�Ϊ��CH4+H2O?CO+3H2��
��4���ںϳɰ���ʵ�����������У���ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪�������������������͵�����Ũ������������Ӧ���ʣ����������͵���Ũ�ȣ����ٰ���Ũ��������ƽ��������У�
�ʴ�Ϊ����������������Ũ������������Ӧ���ʣ���С������Ũ�ȣ���������������Ũ�Ⱦ�������ƽ��������Ӧ�����ƶ���
��5��NaOH��Һ������Ȼ���е���������Na2S�����ʱ���������ӷŵ����ɵ��������������ӷŵ���������������ܷ�Ӧ����ʽΪ��Na2S+2H2O
| ||
�ʴ�Ϊ��Na2S+2H2O
| ||
��6����
| a |
| 17 |
| a |
| 17 |
��4NH3+5O2�T4NO+6H2O��4NO+3O2+2H2O�T4HNO3��
NH3��NO��HNO3 NH3 +HNO3 �TNH4NO3
x mol x mol x mol ��
| a |
| 17 |
�����⣬���̶���ȡNH4NO3����Ӧ�����㣨
| a |
| 17 |
��� x=
| a |
| 34 |
���ԣ���������ʵ���Ũ���ǣ�c��NH4NO3���T
| n |
| V |
| n(�����) | ||
|
| ||||
|
| 500ad |
| 40a+17b |
�ʴ�Ϊ��
| 500ad |
| 40a+17b |
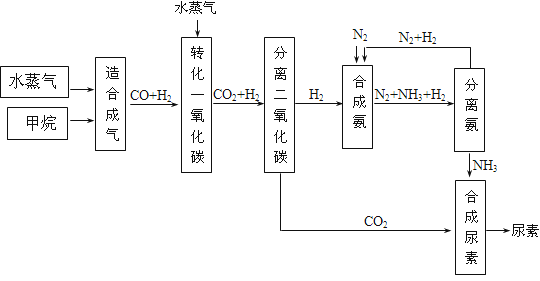
������������һ����ѧ��ҵ�������ϵ���Ŀ���漰��������ԭ��Ӧ������ʽ����д����ѧƽ���ƶ������ԭ�������ݷ���ʽ�ļ���ȣ�����ѧ�������ͽ��������������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
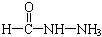

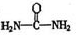 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����
����
��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����
����
��
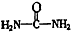 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����__________________ ����_______________________ ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ����__________________ ����_______________________ ��