��Ŀ����
1����1��ʯ�ҹ�ҵ�Թ�ũҵ���������ش�����ʯ�ҵ���Ҫ��ҵ��Ӧ�а��������л�ѧƽ�⣺CaCO3��s��?CaO��s��+CO2��g����H=+178.32kJ/mol����800��ʱ����CO2��g����CaCO3��s����CaO��s��������A��E��ͬ��Ͷ�Ϸ�ʽ������һ��10L���ܱ������У������㹻��ʱ����ά���¶Ȳ��䣬������CaCO3��s�����������ӵ���A�����ٵ���C��D��E����ϵ���ڻ�ѧƽ��״̬����B��������ţ�������֪800��ʱ���÷�Ӧƽ�ⳣ��K=0.003����
| ��� | CaCO3/mol | CaO/mol | CO2/mol |
| A | 0.02 | 0.02 | 0.05 |
| B | 0.02 | 0.02 | 0.03 |
| C | 0.02 | 0.02 | 0.01 |
| D | 0.02 | 0 | 0.05 |
| E | 0.02 | 0.02 | 0 |
CaCO3��s��+H2O��l��+CO2��g��?Ca2+��aq��+2HCO3-��aq����H=-321.7kJ/mol
��һ������c ��Ca2+����c ��HCO3-���ı���ʽ��ʾCa ��HCO3��2 ��Һ�е�c ��OH-������������Ũ�ȼ�Ĺ�ϵ��c ��OH-��=c��H+��+c��H2CO3��-c��CO32-����
��3��Ϊ��ȥ��¯ˮ���к��е�CaSO4��������ij��Һ������ʹ֮ת��Ϊ���ɡ�������������ʣ���ת�������ӷ���ʽ��CaSO4��s��+CO32- ��aq��?CaCO3��s ��+SO42- ��aq����
���� ��1����֪800��ʱ���÷�Ӧƽ�ⳣ��K=0.003������$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$��K��ƽ�������ƶ���CaCO3��s�����������ӣ�$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$=K����ϵ���ڻ�ѧƽ��״̬��$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$��K��ƽ�������ƶ���CaCO3��s����������С��
��2����Һ�д���H2O?H++OH-��HCO3-?H++CO32-��HCO3-+H2O?OH-+HCO3-����Һ��������Դ��ˮ�ĵ��룬��̼�������ˮ�⣬ˮ�����������Ũ��Ϊc��H+��-c��CO32-����̼�����ˮ��õ�������Ũ�ȵ���̼���Ũ�ȣ��ݴ˽��н��
��3��ˮ���к��е�CaSO4��������̼������Һ������ת��Ϊ CaCO3��
��� �⣺��1����֪800��ʱ���÷�Ӧƽ�ⳣ��K=0.003��A��E��ͬ��Ͷ�Ϸ�ʽ������һ��10L���ܱ������У�
A��$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$=$\frac{0.005��0.002}{0.002}$=0.005��0.003��ƽ�������ƶ���CaCO3��s�����������ӣ�
B��$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$=$\frac{0.003��0.002}{0.002}$=0.003=K����ϵ���ڻ�ѧƽ��״̬��
C��$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$=$\frac{0.001��0.002}{0.002}$=0.001��0.003��ƽ�������ƶ���CaCO3��s����������С��
D��$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$=$\frac{0.005��0}{0.002}$=0��0.003��ƽ�������ƶ���CaCO3��s����������С��
E��$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$=$\frac{0��0.002}{0.002}$=0��0.003��ƽ�������ƶ���CaCO3��s����������С��
�ʴ�Ϊ��A��C��D��E��B��
��2����Һ�д���H2O?H++OH-��HCO3-?H++CO32-��HCO3-+H2O?OH-+HCO3-����Һ��������Դ��ˮ�ĵ�����̼�������ˮ�⣬ˮ�����������Ũ��Ϊc��H+��-c��CO32-����̼�����ˮ��õ�������Ũ�ȵ���̼���Ũ�ȣ�����Һ��c��OH-��=c��H+��+c��H2CO3��-c��CO32-����
�ʴ�Ϊ��c��H+��+c��H2CO3��-c��CO32-����
��3��ˮ���к��е�CaSO4��������̼������Һ������ת��Ϊ CaCO3��ת�������ӷ���ʽ��CaSO4��s��+CO32- ��aq��?CaCO3��s ��+SO42- ��aq����
�ʴ�Ϊ��CaSO4��s��+CO32- ��aq��?CaCO3��s ��+SO42- ��aq����
���� ���⿼�黯ѧƽ���Ӱ�����ء�����Ũ�ȵıȽϡ�����ת���ȣ���2��Ϊ�״��㡢�ѵ㣬ѧ��������ˮ����Ŀ�Ѷ��еȣ�

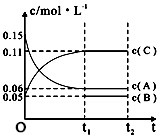
| A�� | ��a=3����b=1��c=2 | |
| B�� | t1minʱ���÷�Ӧ�ﵽ�������µķ�Ӧ�� | |
| C�� | ��O��t1min�ڣ���C��ʾ�Ļ�ѧ��Ӧ����Ϊ0.06mol•L-1 | |
| D�� | B����ʼŨ�ȵ���0.08mol•L-1 |
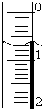 ʹ������к͵ζ����ⶨ���۰״���������g/100mL����
ʹ������к͵ζ����ⶨ���۰״���������g/100mL������ʵ�鲽�裺
��1������100mL����״���Һ����ȡ10.00mLʳ�ð״ף�ע���ձ�����ˮϡ�ͺ�ת�Ƶ�100mL����ƿ�����������ƣ��ж��ݣ�ҡ�ȼ��ã�
��2��ȡ����״���Һ20.00mL����ƿ�У������еμ�2�η�̪��ָʾ����
��3����ȡʢװ0.1000mol/L NaOH ��Һ�ļ�ʽ�ζ��ܣ����������ƣ��ij�ʼ���������Һ��λ������ͼ��ʾ�����ʱ�Ķ���Ϊ0.70mL��
��4���ζ�������Һ����ɫǡ�ñ�Ϊ��ɫ�����ڰ�����ڲ���ɫʱ��ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Σ�
��ʵ���¼
| �ζ����� ʵ�����ݣ�mL�� | 1 | 2 | 3 | 4 |
| V����Ʒ�� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH�������ģ� | 15.95 | 15.00 | 15.05 | 14.95 |
��1�������㣬���۰״�������=4.5g/100mL
��2���ڱ�ʵ��ζ������У����в�����ʹʵ����ƫ����ab����д��ţ�
a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
b����ʽ�ζ��ܼ����ڵζ�ǰ�����ݣ��ζ���������
c����ƿ�м��������Һ���ټ���ˮ
d����ƿ�ڵζ�ʱ����ҡ��������Һ�彦����
| A�� | Fe2++2NH3•H2O�TFe��OH��2��+2NH4+ | |
| B�� | Fe2++NH3•H2O+HCO3-�TFeCO3��+NH4++H20 | |
| C�� | Fe2++2HCO3-�TFe��OH��2��+2CO2�� | |
| D�� | 2Fe2++HCO3-+3NH3•H2O�TFe2��OH��2CO3��+3NH4++H2O |
 ��ش��������⣮
��ش��������⣮��1��ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�
��100��ʱ1mol•L-1��NaOH��Һ�У���ˮ�������c��H+��=1��10-12mol•L-1��KW��25�棩��KW��100�棩�����������������=������
��25��ʱ����ˮ�ĵ���ƽ����ϵ�м�������NH4Cl���壬��ˮ�ĵ���ƽ���Ӱ���Ǵٽ�����ٽ����������ơ���Ӱ�족����
��2������ƽ�ⳣ���Ǻ���������ʵ���̶�ǿ����������֪������ݣ�
| ��ѧʽ | ����ƽ�ⳣ����25�棩 |
| HCN | K=4.9��10-10 |
| CH3COOH | K=1.8��10-5 |
| H2CO3 | K1=4.3��10-7��K2=5.6��10-11 |
��25��ʱ����Ũ�ȵ�CH3COOH��Һ��NaOH��Һ�������ϣ���c��Na+����c��CH3COO-�������������������=������
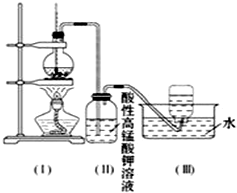 ��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ�����Һ���к�ɫ������֣���һ��ʱ��������ữ�ĸ��������Һ��ɫ����������֪�������������к���CH2=CH2��SO2��CO2��H2O��
��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ�����Һ���к�ɫ������֣���һ��ʱ��������ữ�ĸ��������Һ��ɫ����������֪�������������к���CH2=CH2��SO2��CO2��H2O��
