��Ŀ����
��ˮ�ǻ���ԭ�ϵ���Ҫ��Դ�������ǹ�ҵ�϶Ժ�ˮ�ۺ����õ�ʾ��ͼ��

�ش��������⣺
��1���Ӻ�ˮ�еõ������к�![]() ���������ӣ������Լ�BaCl2��NaOH�������Na2CO3��Һ����ȥ�������ӣ�������Լ���˳����____________________��
���������ӣ������Լ�BaCl2��NaOH�������Na2CO3��Һ����ȥ�������ӣ�������Լ���˳����____________________��
��2���Ժ�̲����Ϊԭ���Ʊ�Ca(OH)2�Ĺ��̿��û�ѧ����ʽ��ʾΪ_____________��___________________��
��3��MgCl2?6H2O��ˮ��������ˮ�����ɼ�ʽ�Ȼ�þ����ѧ����ʽΪ____________����˹�ҵ�ϳ�ʹMgCl2?6H2O ������_______��������ˮ���õ�������ˮ��MgCl2��
��4�������ˮ MgCl2�ɵý���þ�����������ø���Ʒ���������Ʊ�Ư�ۣ��䷴Ӧ�Ļ�ѧ����ʽΪ________________________��
��1��![]() �������NaOH��
�������NaOH��![]() �����ᡣ
�����ᡣ
��2��![]()
![]()
![]()
![]()
��3��![]()
![]()
![]() ���Ȼ��⡡
���Ȼ��⡡
��4��![]()

��ϰ��ϵ�д�
�����Ŀ

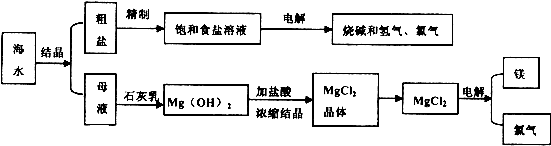




 ���������ӣ������Լ�BaCl2��NaOH�������Na2CO3��Һ����ȥ�������ӣ�������Լ���˳����____________________��
���������ӣ������Լ�BaCl2��NaOH�������Na2CO3��Һ����ȥ�������ӣ�������Լ���˳����____________________��  6H2O��ˮ��������ˮ�����ɼ�ʽ�Ȼ�þ����ѧ����ʽΪ____________����˹�ҵ�ϳ�ʹMgCl2
6H2O��ˮ��������ˮ�����ɼ�ʽ�Ȼ�þ����ѧ����ʽΪ____________����˹�ҵ�ϳ�ʹMgCl2 6H2O ������_______��������ˮ���õ�������ˮ��MgCl2��
6H2O ������_______��������ˮ���õ�������ˮ��MgCl2��