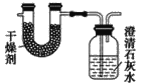
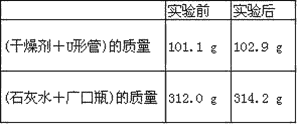
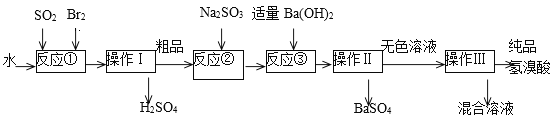
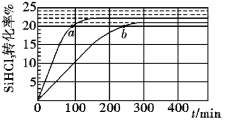
��Ŀ����
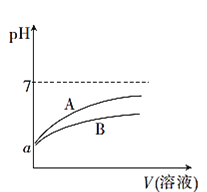
����Ŀ��25��ʱ���ֱ�������pH��Ϊa��CH3COOH��Һ��HCN��Һ�м�ˮϡ�ͣ�ϡ��������Һ��pH�仯����Һ����Ĺ�ϵ��ͼ��ʾ��
��֪25��ʱ��HCN�ĵ���ƽ�ⳣ��Ka=6.2��10-10��CH3COOH�ĵ���ƽ�ⳣ��Ka=1.7��10-5
(1)��ʾCH3COOH��Һ��pH�仯���Ƶ�������____(����A������B��)��
(2)pH��Ϊa��CH3COOH��Һ��HCN��Һ�����ʵ����ʵ���Ũ�Ƚϴ����____(�ѧʽ)��
(3)25��ʱ����Ũ�ȵ�NaCN��Һ��pH___����>��=������<��)CH3 COONa��Һ��pH��
(4)25��ʱ����20mL0.01mol��L-1CH3COOH��Һ����μ���0.01mol��L-1KOH��Һ����pH=7ʱ������KOH��Һ�����____(�����<��)20mL��
(5)����ͬŨ�ȵ�AgNO3��Һ�ֱ�ζ�Ũ����ͬ�ĺ�Cl����Br����I���Ļ����Һ����ͼ��ȷ�����ȳ�����������______��(�����ӷ���)
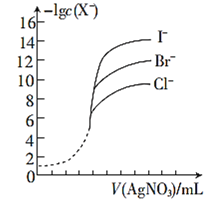
���𰸡�A HCN > < I-
��������
��֪25��ʱ��HCN�ĵ���ƽ�ⳣ��Ka=6.2��10-10��CH3COOH�ĵ���ƽ�ⳣ��Ka=1.7��10-5��HCN�ĵ���ƽ�ⳣС��CH3COOH�ĵ���ƽ�ⳣ������CH3COOH�����Ա�HCN������ǿ������Խǿ�����Ӧ��������ӵ�ˮ������Խ���������ܽ�ƽ���Ӧ�á�
��1��CH3COOH�����Ա�HCN������ǿ��pH���ʱ����ˮϡ����ͬ�ı��������Խ�ǿ��pH�仯��A��ʾCH3COOH��Һ��pH�仯���Ƶ����ߣ�
��2������HCN�ĵ���ƽ�ⳣС��CH3COOH�ĵ���ƽ�ⳣ���������ʵ���Ũ����ȵ�CH3COOH��Һ��HCN��Һ��CH3COOH����Һ�е������c(H+)������HCN����Һ�е������c(H+)����pH��ȵ�CH3COOH��Һ��HCN��Һ�����ʵ����ʵ���Ũ�Ƚϴ����HCN��Һ��
��3������Խǿ�����Ӧ��������ӵ�ˮ������Խ��������CH3COOH�����Ա�HCN������ǿ������CN-��ˮ��������CH3COO-��ˮ��ˮ��ǿ����25��ʱ����Ũ�ȵ�NaCN��Һ��pH>CH3COONa��Һ��pH��
��4��25��ʱ����20mL0.01mol��L-1CH3COOH��Һ����μ���0.01mol��L-1KOH��Һ��������KOH��Һ�����Ϊ20mLʱ��CH3COOH��KOHǡ����ȫ��Ӧ����CH3COOK��CH3COOK��ǿ�������Σ���Һ�ʼ��ԣ��ʵ�pH=7ʱ������KOH��Һ�����<20mL��
��5����ͼ���֪-lgc(X-)��ֵԽ����c(X-)ԽС����c(Ag+)��ͬʱ����ʼ��������c(I-)��С��������ͬŨ�ȵ�AgNO3��Һ�ֱ�ζ�Ũ����ͬ�ĺ�Cl����Br����I���Ļ����Һ�����ȳ�����������I- ��