��Ŀ����
�״���һ������ȼ�ϣ�������ȼ�ϵ�أ�
��1����ҵ�Ͽ����������ַ�Ӧ�Ʊ��״���CO��g��+2H2��g��?CH3OH��g����H1��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H2
��֪��2H2��g��+O2��g���T2H2O��g����H3����2CO��g��+O2��g���T2CO2��g���ķ�Ӧ�ȡ�H= ���á�H1����H2����H3��ʾ����
��2�������״���ԭ��CO��H2��Դ�ڣ�CH4��g��+H2O��g��?CO��g��+3H2��g����H4��һ��������CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼa�����H4 0��P1 P2�����������������=����

��3��ijʵ��С�������ͼb��ʾ�ļ״�ȼ�ϵ��װ�ã�����һ��ʱ�����Һ��PH ��������С�����䣩�������ĵ缫��ӦʽΪ ��
��4����ͭ��������õ��Ĵ�ͭ������Fe��Ag��Au�Ƚ������ʣ����һ�����õ�ⷨ���ƣ���ͭ���õ���ͭ�ĵ����У����������� ������������ ��������Ϊ�� ��
��1����ҵ�Ͽ����������ַ�Ӧ�Ʊ��״���CO��g��+2H2��g��?CH3OH��g����H1��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H2
��֪��2H2��g��+O2��g���T2H2O��g����H3����2CO��g��+O2��g���T2CO2��g���ķ�Ӧ�ȡ�H=
��2�������״���ԭ��CO��H2��Դ�ڣ�CH4��g��+H2O��g��?CO��g��+3H2��g����H4��һ��������CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼa�����H4

��3��ijʵ��С�������ͼb��ʾ�ļ״�ȼ�ϵ��װ�ã�����һ��ʱ�����Һ��PH
��4����ͭ��������õ��Ĵ�ͭ������Fe��Ag��Au�Ƚ������ʣ����һ�����õ�ⷨ���ƣ���ͭ���õ���ͭ�ĵ����У�����������
���㣺�ø�˹���ɽ����йط�Ӧ�ȵļ���,ͭ�ĵ�⾫��,��ѧ��Դ���͵��,ת�������¶ȡ�ѹǿ�ı仯����
ר�⣺��ѧ��Ӧ�е������仯,��ѧƽ��ר��,�绯ѧר��
��������1�����ø�˹���ɽ��ע���������Ӧ�еķ�Ӧ�������������֪��Ӧ�е�λ�ã�ͨ���Ӽ���ã�
��2����COת�������¶ȵı仯���Ʒ�����һ�ʣ���200��ʱ������ͬѹǿ��CO��ת���ʴ�С����ϻ�ѧ����ʽ�з�Ӧǰ�����������仯���
��3����ȼ�ϵ�ص��ܷ�ӦΪ2CH3OH+3O2+4OH-�T2CO32-+6H2O������һ��ʱ���c��OH-����С��PH��С��������ӦʽΪO2+2H2O+4e-=4OH-���ܷ�Ӧʽ��ȥ������Ӧʽ�ø÷�Ӧ�ĸ�����ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O��
��4�����ݵ��ԭ�����������ʱ����ͭ����������ͭ���������������ҺΪ����ͭ���ӵ�����Һ�������Ϸ���������Ӧ�������ϵõ��ӷ�����ԭ��Ӧ�����ݴ�ͭ�л�ԭ�ԵĴ�С˳�����������ijɷ֣�
��2����COת�������¶ȵı仯���Ʒ�����һ�ʣ���200��ʱ������ͬѹǿ��CO��ת���ʴ�С����ϻ�ѧ����ʽ�з�Ӧǰ�����������仯���
��3����ȼ�ϵ�ص��ܷ�ӦΪ2CH3OH+3O2+4OH-�T2CO32-+6H2O������һ��ʱ���c��OH-����С��PH��С��������ӦʽΪO2+2H2O+4e-=4OH-���ܷ�Ӧʽ��ȥ������Ӧʽ�ø÷�Ӧ�ĸ�����ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O��
��4�����ݵ��ԭ�����������ʱ����ͭ����������ͭ���������������ҺΪ����ͭ���ӵ�����Һ�������Ϸ���������Ӧ�������ϵõ��ӷ�����ԭ��Ӧ�����ݴ�ͭ�л�ԭ�ԵĴ�С˳�����������ijɷ֣�
���
�⣺��1����CO��g��+2H2��g��?CH3OH��g����H1
��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H2
��2H2��g��+O2��g��=2H2O��g����H3
�ݸ�˹���ɣ���-�ڵã���CO��g��+H2O��g��?CO2��g��+H2��g����H=��H1-��H2
�٢ܡ�2+�۵ã�2CO��g��+O2��g��=2CO2��g����H=2��H1-2��H2+��H3
�ʴ�Ϊ��2��H1-2��H2+��H3��
��2���ٴ�ͼa�ɼ���CH4ת�������¶ȵ����߶�����˵������ʱƽ�������ƶ�����H��0��
��ͼa��200��λ�ã�ƽ�������ửһ�����ߣ��ɼ�CH4��ת����P1��P2����CH4��g��+H2O��g��?CO��g��+3H2��g����Ӧ�У������������������ڷ�Ӧ�ѹǿ����ʱƽ���������ƶ�����P1��P2
�ʴ�Ϊ����������
��3����ȼ�ϵ�ص��ܷ�ӦΪ2CH3OH+3O2+4OH-�T2CO32-+6H2O������һ��ʱ���c��OH-����С��PH��С��������ӦʽΪO2+2H2O+4e-=4OH-���ܷ�Ӧʽ��ȥ������Ӧʽ�ø÷�Ӧ�ĸ�����ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O��
�ʴ�Ϊ����С��CH3OH+8OH--6e-=CO32-+6H2O��
��4�����ݵ��ԭ�����������ʱ����ͭ����������ͭ���������������ҺΪ����ͭ���ӵ�����Һ�������Ϸ���������Ӧ�����ݴ�ͭ�л�ԭ�ԣ�Fe��Cu��Ag��Au��Fe��Cu�������ŵ磬������ijɷ�ΪAg��Au��
�ʴ�Ϊ����ͭ����ͭ��Ag��Au��
��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H2
��2H2��g��+O2��g��=2H2O��g����H3
�ݸ�˹���ɣ���-�ڵã���CO��g��+H2O��g��?CO2��g��+H2��g����H=��H1-��H2
�٢ܡ�2+�۵ã�2CO��g��+O2��g��=2CO2��g����H=2��H1-2��H2+��H3
�ʴ�Ϊ��2��H1-2��H2+��H3��
��2���ٴ�ͼa�ɼ���CH4ת�������¶ȵ����߶�����˵������ʱƽ�������ƶ�����H��0��
��ͼa��200��λ�ã�ƽ�������ửһ�����ߣ��ɼ�CH4��ת����P1��P2����CH4��g��+H2O��g��?CO��g��+3H2��g����Ӧ�У������������������ڷ�Ӧ�ѹǿ����ʱƽ���������ƶ�����P1��P2
�ʴ�Ϊ����������
��3����ȼ�ϵ�ص��ܷ�ӦΪ2CH3OH+3O2+4OH-�T2CO32-+6H2O������һ��ʱ���c��OH-����С��PH��С��������ӦʽΪO2+2H2O+4e-=4OH-���ܷ�Ӧʽ��ȥ������Ӧʽ�ø÷�Ӧ�ĸ�����ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O��
�ʴ�Ϊ����С��CH3OH+8OH--6e-=CO32-+6H2O��
��4�����ݵ��ԭ�����������ʱ����ͭ����������ͭ���������������ҺΪ����ͭ���ӵ�����Һ�������Ϸ���������Ӧ�����ݴ�ͭ�л�ԭ�ԣ�Fe��Cu��Ag��Au��Fe��Cu�������ŵ磬������ijɷ�ΪAg��Au��
�ʴ�Ϊ����ͭ����ͭ��Ag��Au��
���������⿼���˹���ɡ����ԭ����������ͼ������Լ���ѧ��Ӧԭ����Ӧ�ã�Ϊ�߿��ؿ��Ŀ��㣬�Ѷ��еȣ�ѧϰ����ؼ�ǿ��ѧ��Ӧԭ����ѧϰ���о�����˼��ֱ������Ӧ�ã������ۻ�

��ϰ��ϵ�д�
�����Ŀ
��֪�����Ȼ�ѧ����ʽ��Zn��s��+
O2��g��=ZnO��s����H=-351.1kJ?mol-1 Hg��l��+
O2��g��=HgO��s����H=-90.7kJ?mol-1�ɴ˿�֪��ӦZn��s��+HgO��s��=ZnO��s��+Hg��l�����ʱ�Ϊ��������
| 1 |
| 2 |
| 1 |
| 2 |
| A��-260.4 kJ?mol-1 |
| B��-441.8 kJ?mol-1 |
| C��260.4 kJ?mol-1 |
| D��441.8 kJ?mol-1 |
�������û�з��������ʱ��Ե��ǣ�������
| A���ø������ֽ��ƶ���걾 |
| B����պ����������Ϊ75%�ľƾ������Ƥ�� |
| C���������ߵƹ����䲡�� |
| D��������Һ�мӱ���ʳ��ˮ����� |
������ʵ���У�������������ȷ���ǣ�������
| A����������ƿ�з��뼸�����Ƭ����ֹҺ�屩�� |
| B�����¶ȼ�ˮ�������������ƿ֧�ܿڸ��� |
| C����ˮ���������¿��룬�Ͽڳ� |
| D��������ƿ���õ�ʯ��������ֱ�Ӽ��� |



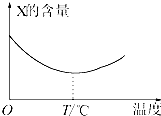 ������̬����X��Y��ֱ�ӻ�������Z�����淴Ӧ�����罫X��Y��һ��������ϲ�ѹ���ܱ������У��ڲ�ͬ�¶��¾���һ��ʱ���Ӧ�������X�ĺ����仯��ͼ��ʾ���ش��������⣺
������̬����X��Y��ֱ�ӻ�������Z�����淴Ӧ�����罫X��Y��һ��������ϲ�ѹ���ܱ������У��ڲ�ͬ�¶��¾���һ��ʱ���Ӧ�������X�ĺ����仯��ͼ��ʾ���ش��������⣺ ij��ʳƷ�����ϱ�ǩ��ͼ��ʾ��
ij��ʳƷ�����ϱ�ǩ��ͼ��ʾ��