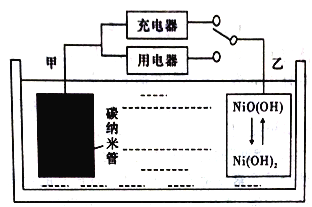
��Ŀ����
����Ŀ��������������Ҫ����A12O3��Fe2O3��FeO��SiO2���������Ʊ���ˮ������KAl(SO4)2��12H2O�Ͳ�Ѫ��FeSO4��7H2O��������������(���ֲ����Ͳ�����ȥ)��
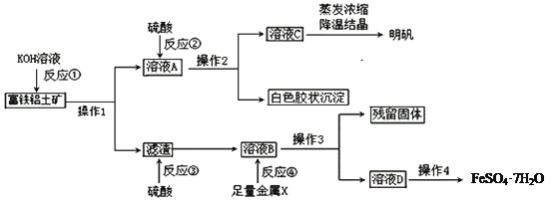
(1)����1����Ҫ�õ��IJ�������______________________��
(2)��Ӧ�٢ڢۢ�����������ԭ��Ӧ����_________����д��ţ���
(3)�ۺϿ��ǣ�����X���ѡ��__________��д���÷�Ӧ���ӷ�����ʽ_________________________��
(4)��Ӧ�ٵ����ӷ���ʽ��___________________��___________________________��
(5)��ҺD�к��еĽ�����������_______�����鷽����_______________________________��
���𰸡���ͨ©�������������ձ� �� Fe Fe��2Fe3+ = 3Fe2+ SiO2��2OH�� = SiO32-��H2O Al2O3��2OH�� = 2AlO2-��H2O Fe2+ ȡ������ҺD���Թ��У��������軯����Һ���������ٵ��뼸����ˮ������죬֤������Fe2+ ��(�������������Һ���۲쵽���ɳ�������ɫ�ɰ�ɫѸ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��֤������Fe2+��)
��������
������������A12O3��SiO2��KOH��Һ��Ӧ����ƫ����غ���أ����˷���Ϊ����Fe2O3��FeO��ƫ����ء�����صĻ����Һ����Һ�м����������˵õ�����Ľ�״������������������صĻ����Һ��������������صĻ����Һ����Ũ�������½ᾧ�õ��������壻����Fe2O3��FeO�����ᷴӦ���������������������������м�����������ۣ���������Ӧȫ�������������������˵���������������Ũ������ȴ�ᾧ�����ɲ�Ѫ��FeSO4��7H2O��
�� ����1Ϊ���ˣ�������Ҫ������Ϊ��ͨ©�������������ձ����ʴ�Ϊ����ͨ©�������������ձ���
�� �ܵķ�Ӧ������ԭFe3+�����������ӷ�Ӧ����ʽ��2Fe3++Fe= 3Fe2+������٢ڢ۾�Ϊ���ֽⷴӦ���ʴ�Ϊ���ܣ�
�� ���ڲ��������������������ӣ����Խ���X���ѡ��Fe�����������ӷ�Ӧ����ʽ��2Fe3++Fe= 3Fe2+���ʴ�Ϊ��Fe��2Fe3++Fe= 3Fe2+��
�� ������������A12O3��SiO2��KOH��Һ��Ӧ�����ӷ�Ӧ����ʽΪAl2O3 + 2OH- = 2AlO2- + H2O��SiO2 + 2OH- = SiO32- + H2O���ʴ�Ϊ��Al2O3 + 2OH- = 2AlO2- + H2O��SiO2 + 2OH- = SiO32- + H2O��
�� ��ҺD�к��еĽ�����������Fe2��������Fe2���Ļ�ԭ�Լ���Fe2+��������Fe2+���ķ�Ӧ���飩�����������ȡ������ҺD���Թ��У��������軯����Һ���������ٵ��뼸����ˮ������죬֤������Fe2��(�������������Һ���۲쵽���ɳ�������ɫ�ɰ�ɫѸ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��֤������Fe2+)���ʴ�Ϊ��ȡ������ҺD���Թ��У��������軯����Һ���������ٵ��뼸����ˮ������죬֤������Fe2+(�������������Һ���۲쵽���ɳ�������ɫ�ɰ�ɫѸ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��֤������Fe2+)��