��Ŀ����
Ԫ�صķ�������
��1��s ���� �����ĵ�������_______�ϣ�����_________�����ڻ��ý�����Ϊ������ͼ���������
�����ĵ�������_______�ϣ�����_________�����ڻ��ý�����Ϊ������ͼ���������
��2��p���� �����ĵ�������_______�ϣ�����_______��Ԫ�أ�Ϊ�ǽ���������������
�����ĵ�������_______�ϣ�����_______��Ԫ�أ�Ϊ�ǽ���������������
��3��d���� �����ĵ�������_______�ϣ�����_______��Ԫ�أ�Ϊ���ɽ�����
�����ĵ�������_______�ϣ�����_______��Ԫ�أ�Ϊ���ɽ�����
��4��ds���� �� (n-1)dȫ������ ���ĵ�������_______�ϣ�����_______�����ɽ���(d��ds��������������Ϊ���ɽ���)��
�� (n-1)dȫ������ ���ĵ�������_______�ϣ�����_______�����ɽ���(d��ds��������������Ϊ���ɽ���)��
��5��f���� ������_______Ԫ�أ���Ϊ�ڹ���Ԫ�ػ��ڹ���ϵ��
������_______Ԫ�أ���Ϊ�ڹ���Ԫ�ػ��ڹ���ϵ��
��1��s ����
 �����ĵ�������_______�ϣ�����_________�����ڻ��ý�����Ϊ������ͼ���������
�����ĵ�������_______�ϣ�����_________�����ڻ��ý�����Ϊ������ͼ�����������2��p����
 �����ĵ�������_______�ϣ�����_______��Ԫ�أ�Ϊ�ǽ���������������
�����ĵ�������_______�ϣ�����_______��Ԫ�أ�Ϊ�ǽ�����������������3��d����
 �����ĵ�������_______�ϣ�����_______��Ԫ�أ�Ϊ���ɽ�����
�����ĵ�������_______�ϣ�����_______��Ԫ�أ�Ϊ���ɽ�������4��ds����
 �� (n-1)dȫ������ ���ĵ�������_______�ϣ�����_______�����ɽ���(d��ds��������������Ϊ���ɽ���)��
�� (n-1)dȫ������ ���ĵ�������_______�ϣ�����_______�����ɽ���(d��ds��������������Ϊ���ɽ���)�� ��5��f����
 ������_______Ԫ�أ���Ϊ�ڹ���Ԫ�ػ��ڹ���ϵ��
������_______Ԫ�أ���Ϊ�ڹ���Ԫ�ػ��ڹ���ϵ�� ��1��ns��IA��IIA��Ԫ��
��2��np��IIIA-VIIA�塢����Ԫ��
��3��(n-1)d����IIIB�嵽VIII��Ԫ��
��4��ns��IB���IIB��Ԫ��
��5����ϵ���ϵԪ��
��2��np��IIIA-VIIA�塢����Ԫ��
��3��(n-1)d����IIIB�嵽VIII��Ԫ��
��4��ns��IB���IIB��Ԫ��
��5����ϵ���ϵԪ��

��ϰ��ϵ�д�
�����Ŀ
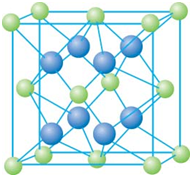 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B�γɵľ����侧���ṹ��ͼ��ͼ��С�����D���������B����ش�
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B�γɵľ����侧���ṹ��ͼ��ͼ��С�����D���������B����ش�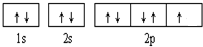
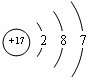
 ��2��ƫ���ᱵ��С�ͱ�ѹ������Ͳ���������ж���Ӧ�ã�ƫ���ᱵ�����о����Ľṹ����ͼ��ʾ�����Ļ�ѧʽ��
��2��ƫ���ᱵ��С�ͱ�ѹ������Ͳ���������ж���Ӧ�ã�ƫ���ᱵ�����о����Ľṹ����ͼ��ʾ�����Ļ�ѧʽ��