��Ŀ����
10����һ����ɫ�����ĩ�����ܺ���K2SO4��Na2CO3��NH4Cl��BaCl2��CuSO4�е�һ�ֻ��֣������²������ʵ�飺��ȡ���������ĩ�ӵ�����ˮ�У��õ���ɫ��Һ��
��ȡ������Һ�������������м�������ϡ���ᣬ�����ݲ�����
�ۼ�������е���Һ�μ�Ba��NO3��2��Һ���а�ɫ�������ɣ�
��ȡ������Һ����������NaOH��Һ�����ȣ������̼�����ζ�����壬����ʪ��ĺ�ɫʯ����ֽ�������壬��ֽ������
����������ʵ�жϣ�
��1���϶����ڵ���Na2CO3��NH4Cl��
��2���϶������ڵ���BaCl2��CuSO4��
��3������ȷ���Ƿ���ڵ���K2SO4�������֤���������Ƿ���ڵ�ʵ�鷽����ʵ�鷽���������ۣ��ýྻ��˿պȡ����������Һ�������գ�����ɫ�ܲ����۲쵽�������ɫ��˵����K2SO4������û�У�����ȡ������Һ�������ữ���������ɣ��ټ�������BaCl2��Һ���а�ɫ�������ɣ�˵��ԭ��Ʒ����K2SO4������û�У�
��4��д������ʵ����в����̼�����ζ���巴Ӧ�Ļ�ѧ����ʽ��NH4Cl+NaOH$\frac{\underline{\;\;��\;\;}}{\;}$NaCl+NH3��+H2O��
���� ������Ŀ��������Ϣ����ȡ���������ĩ�ӵ�����ˮ�У��õ���ɫ��Һ�����ų�CuSO4��ͬʱ����BaCl2��Na2CO3��K2SO4�����棬��ȷ������BaCl2���ڣ����ų����ߣ���֮��BaCl2�����ڣ���ȡ������Һ�������������м�������ϡ���ᣬ�����ݲ�������֪���ɵ�����Ϊ������̼��ȷ����Һ����Na2CO3���ų�BaCl2�Ĵ��ڣ��ۼ�������е���Һ�μ�Ba��NO3��2��Һ���а�ɫ�������ɣ��˳���ΪBaSO4������ȷ��ԭ�������K2SO4��ԭ��ʵ������������ữ������SO42-����ȡ������Һ����������NaOH��Һ�����ȣ������̼�����ζ�����壬����ʪ��ĺ�ɫʯ����ֽ�������壬��ֽ��������֪���ɵ�����ΪNH3��ȷ����Һ����NH4+������NH4Cl���ݴ˷�����֪��ԭ�������һ����NH4Cl��Na2CO3��һ��û��CuSO4��BaCl2��������K2SO4��
��� �⣺��1����ʵ��ڿ�֪��������Na2CO3��ʵ��ܿ�֪��������NH4Cl���ʴ�Ϊ��NH4Cl��Na2CO3��
��2����ʵ����ų���CuSO4������Na2CO3�ų�BaCl2���ʴ�Ϊ��CuSO4��BaCl2��
��3����ȷ������K2SO4����������ɫ��Ӧ���ܹ�SO42-�ļ��飬ȷ���Ƿ���ڣ��������Ϊ���ýྻ��˿պȡ����������Һ�������գ�����ɫ�ܲ����۲쵽�������ɫ��˵����K2SO4������û�У� ����ȡ������Һ�������ữ���������ɣ��ټ�������BaCl2��Һ���а�ɫ�������ɣ�˵��ԭ��Ʒ����K2SO4������û�У��ʴ�Ϊ���ýྻ��˿պȡ����������Һ�������գ�����ɫ�ܲ����۲쵽�������ɫ��˵����K2SO4������û�У� ����ȡ������Һ�������ữ���������ɣ��ټ�������BaCl2��Һ���а�ɫ�������ɣ�˵��ԭ��Ʒ����K2SO4������û�У�
��4��ʵ����в����̼�����ζ���巴Ӧ�Ļ�ѧ����ʽΪNH4Cl+Na OH$\frac{\underline{\;\;��\;\;}}{\;}$Na Cl+NH3��+H2O���ʴ�Ϊ��NH4Cl+Na OH$\frac{\underline{\;\;��\;\;}}{\;}$Na Cl+NH3��+H2O��
���� ���������ƶ��⣬�������������͵��ƶ��⣬��ͨ����ʵ�鷽�����̵�̽�����ڱȽϼ���Ļ����ϣ��ó�����ȷ��ʵ����ۣ����������п�����Ҫ����֮һ��Ҫ��ϸ���⣬������գ��˿�����Ҫ������������ʵ������

| A�� | 0.3 mol/L | B�� | 0.03 mol/L | C�� | 0.05 mol/L | D�� | 0.04 mol/L |
| A�� | �۳� | B�� | �����ЧӦ | C�� | �����˶� | D�� | ������ |
| A�� | ұ��������ͨ���õ���Ȼ�����Һ�ķ��� | |
| B�� | Ҫʹ��ˮ����ͨ�����������ӽ����� | |
| C�� | ������������������Ư�� | |
| D�� | ��ҵұ�����������������������������·������ȷ�Ӧ |
| A�� | H2O ��g���TH2��g��+$\frac{1}{2}$O2��g������H�T-485 kJ/mol | B�� | 2H2��g��+O2��g���T2H2O��g������H�T-485 kJ/mol | ||
| C�� | 2H2��g��+O2 ��g���T2H2O��g������H�T+485 kJ/mol | D�� | H2O ��g���TH2��g��+$\frac{1}{2}$O2��g������H�T+485 kJ/mol |
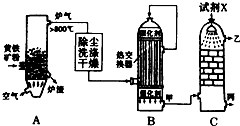 �ϳɰ���ҵ�����Ṥҵ�����Ṥҵ�ǻ�ѧ��ҵ����Ҫ��ɲ��֣���ش��������⣺
�ϳɰ���ҵ�����Ṥҵ�����Ṥҵ�ǻ�ѧ��ҵ����Ҫ��ɲ��֣���ش��������⣺