��Ŀ����
ͨ����ʳ���Ϳɻ��ij�����л�������X,����Է�������Ϊ46,����̼����������Ϊ52.2%,�����������Ϊ13.0%��
(1)X�ķ���ʽ������������
(2)X������Ʒ�Ӧ�ų�����,��Ӧ�Ļ�ѧ����ʽ������������
(3)X������е�������ͭ�������·�Ӧ����Y,Y�Ľṹ��ʽ������������
(4)X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ����������,X��Z��Ӧ������һ������ζ������W,��184 g X��120 g Z��Ӧ������106 g W,����÷�Ӧ�IJ���(Ҫ��д���������)��
(1)C2H6O(2��)
(2)2Na+2CH3CH2OH 2CH3CH2ONa+H2��(3��)
2CH3CH2ONa+H2��(3��)
(3)CH3CHO(2��)
(4)60.2%(3��)
����

���罫������ǰ�������Ծ����һ�ţ�����װ��ϡ������ձ��У�������ַ�Ӧ���ձ��в�������������ʲô��
| A������ | B�������� | C������ | D������ |
I����ϩ��ʯ���ѽ�������Ҫ�ɷ֣����IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����ش��������⡣
(1)��ϩ�Ľṹ��ʽΪ____________��
(2)���������У�������ͨ����ϩ�ӳɷ�Ӧ�õ�����______(�����)��
| A��CH3CH3 | B��CH3CHCl2 |
| C��CH3CH2OH | D��CH3CH2Br |
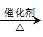 2CH3COOH��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ�·������ͼ��ʾ��
2CH3COOH��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ�·������ͼ��ʾ��
��Ӧ�ڵĻ�ѧ����ʽΪ____________________________________��
��ҵ������ϩΪԭ�Ͽ�������һ����Ҫ�ĺϳ��л��߷��ӻ�����䷴Ӧ�Ļ�ѧ����ʽΪ____________________________________����Ӧ������__________________��
II����ʵ���ҿ���������ͼ��ʾ��װ����ȡ������������ش��������⡣

(1)�Ҵ�����������й����ŵ����Ʒֱ���_________��_________��
(2)�Թ�a�з�����Ӧ�Ļ�ѧ����ʽΪ
_____________________________________________��
��Ӧ������______________��
��3��ŨH2SO4�������ǣ�__________________________��
��Ӧ��ʼǰ���Թ�b��ʢ�ŵ���Һ��____________��
����Һ��������
___________________________________________________________________________________��
��ѧʽΪC2H6O�Ļ�����A�����������ʣ�A��Na�D��������������
A��CH3COOH ����ζ�IJ���
����ζ�IJ���
��1������������Ϣ���Ըû�������������ж���
����������
| A��һ�����С�OH | B��һ�����С�COOH |
| C��AΪ�Ҵ� | D��AΪ��ȩ |
��3��A���Ʒ�Ӧ�Ļ�ѧ����ʽ��_____________��
��4��������A��CH3COOH��Ӧ���ɵ�����ζ�IJ���Ľṹ��ʽΪ__________��



