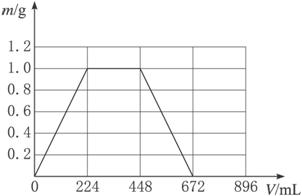
��Ŀ����
���ʱ������CO2�����Ϊ224mL����״��������CO2����ˮ��������������ͬ����
��1�����ɳ����������պ����ʱ����Ӧ�ķ���ʽΪ��
��2��ԭ�������Ca��OH��2������Ϊ
��3������ǡ���ܽ�ʱ������CO2�����Ϊ
��4��д����Һ�����ɳ���������m��g����ͨ��CO2�����V��mL��֮��ĺ�������ʽ��
| V��ȡֵ��Χ | m=f��V�� |

��2OH-+CO2=CO32-����CO32-+Ca2+=CaCO3����CaCO3+CO2+H2O=Ca��HCO3��2��
�������Ϸ�Ӧ���ɰѷ�Ӧ��Ϊ�ĸ��Σ�
��1��������̼���Ⱥ��������Ʒ�Ӧ����̼��Ƴ���������ʽΪCO2+Ca��OH��2=CaCO3��+H2O��
��2��������̼��������������ط�Ӧ������ʽΪ2K0H+CO2=K2CO3+H2O��
��3��������̼�������̼��ط�Ӧ������ʽΪK2CO3+CO2+H2O=2KHCO3��
��4��������̼�������̼��Ʒ�Ӧ����̼����ƣ�����ʽΪCaCO3+CO2+H2O=Ca��HCO3��2��
������CO2�����Ϊ224mL��ϻ�ѧ����ʽ���㣮
�ʴ�Ϊ��CO2+Ca��OH��2=CaCO3��+H2O��
��2�����ݷ�Ӧ����ʽΪCO2+Ca��OH��2=CaCO3��+H2O��
���������Ƶ����ʵ���Ϊx����ôxӦ�õ��ڶ�����̼��������
| 0.224L |
| 22.4L/mol |
�����������Ƶ�����Ϊ0.01mol��74g/mol=0.74g��
�ʴ�Ϊ��0.74��
��3��1.86gKOH��Ca��OH��2�������n��KOH��=
| 1.86g-0.74g |
| 56g/mol |
����ǡ���ܽ�ʱ������Ӧ�ķ�Ӧ�Դ��У�
��CO2+Ca��OH��2=CaCO3��+H2O
0.01mol 0.01mol
��2K0H+CO2=K2CO3+H2O
0.02mol 0.01mol
��K2CO3+CO2+H2O=2KHCO3��
0.01mol 0.01mol
��CaCO3+CO2+H2O=Ca��HCO3��2
0.01mol 0.01mol
����ǡ���ܽ�ʱ������CO2�����ʵ���Ϊ0.01mol+0.01mol+0.01mol+0.01mol=0.04mol��
�����0.04mol��22.4L/mol=0.896L����896ml��
�ʴ�Ϊ��896��
3���ٵ�V��224mlʱ����ӦΪCO2+Ca��OH��2=CaCO3��+H2O��
| Vml |
| 22400ml/mol |
| mg |
| 100g/mol |
| V |
| 224 |
�ڵ�224��V��672mlʱ����ӦΪ��CO2+Ca��OH��2=CaCO3��+H2O����2K0H+CO2=K2CO3+H2O��K2CO3+CO2+H2O=2KHCO3��
m=0.01mol��100g/mol=1g��
�۵�672��V��896Lʱ����ӦΪCaCO3+CO2+H2O=Ca��HCO3��2��
| (V-672)ml |
| 22400ml/mol |
| (1-m)g |
| 100g/mol |
| V-672 |
| 224 |
�ܵ�V��896mlʱ��m=0g������ʽΪK2CO3+CO2+H2O=2KHCO3
�ʴ�Ϊ��
| V��ȡֵ��Χ | m=f��V�� |
| 0��V��224 | m=V/224 |
| 224��V��672 | m=1 |
| 672��V��896 | m=1-��V-672��/224 |
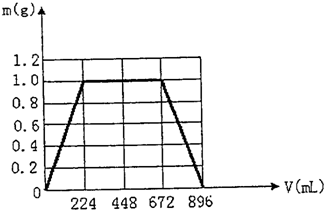 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�(10��) ��KOH��Ca��OH��2�����1.86gȫ������һ����ˮ���γ�ϡ��Һ���ٻ���ͨ��������CO2���塣�����ɳ����������պ����ʱ������CO2�����Ϊ224mL����״��������CO2����ˮ��������������ͬ����
��1�����ɳ����������պ����ʱ����Ӧ�ķ���ʽΪ�� __________________
��2��ԭ�������Ca��OH��2������Ϊ _________ g��
��3������ǡ���ܽ�ʱ������CO2�����Ϊ __________ mL
��4��д����Һ�����ɳ���������m��g����ͨ��CO2�����V��mL��֮��ĺ�������ʽ��
| V��ȡֵ��Χ | m=f��V�� |
|
|
|
|
|
|
|
|
|
��5����ͼʾ����ϵ�ϣ��������ɳ���������m��g����ͨ��CO2�����V��mL���Ĺ�ϵ���ߡ�
(10��) ��KOH��Ca��OH��2�����1.86gȫ������һ����ˮ���γ�ϡ��Һ���ٻ���ͨ��������CO2���塣�����ɳ����������պ����ʱ������CO2�����Ϊ224mL����״��������CO2����ˮ��������������ͬ����


��1�����ɳ����������պ����ʱ����Ӧ�ķ���ʽΪ�� __________________
��2��ԭ�������Ca��OH��2������Ϊ _________ g��
��3������ǡ���ܽ�ʱ������CO2�����Ϊ __________ mL
��4��д����Һ�����ɳ���������m��g����ͨ��CO2�����V��mL��֮��ĺ�������ʽ��
| V��ȡֵ��Χ | m=f��V�� |
| | |
| | |
| | |




(10��) ��KOH��Ca��OH��2�����1.86gȫ������һ����ˮ���γ�ϡ��Һ���ٻ���ͨ��������CO2���塣�����ɳ����������պ����ʱ������CO2�����Ϊ224mL����״��������CO2����ˮ��������������ͬ����
��1�����ɳ����������պ����ʱ����Ӧ�ķ���ʽΪ�� __________________
��2��ԭ�������Ca��OH��2������Ϊ _________ g��
��3������ǡ���ܽ�ʱ������CO2�����Ϊ __________ mL
��4��д����Һ�����ɳ���������m��g����ͨ��CO2�����V��mL��֮��ĺ�������ʽ��
|
V��ȡֵ��Χ |
m=f��V�� |
|
|
|
|
|
|
|
|
|
��5����ͼʾ����ϵ�ϣ��������ɳ���������m��g����ͨ��CO2�����V��mL���Ĺ�ϵ���ߡ�