��Ŀ����
��ҵ�����������ᣨ�е㣺90oC��ʱ��ͬʱ�������������ƣ��乤���������£�
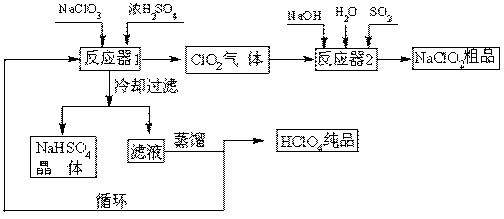
��1����ȴ���˵�Ŀ�� ��
��2��ͨ�뷴Ӧ��2��SO2������ ����Ӧ��2�з�����Ӧ�����ӷ���ʽΪ ��
��3��ѭ��ʹ�õ������� ��
��4������ͨ��������Һ�ķ����õ��������ԭ������� ��
��5��ͨ�����NaClO3ˮ��Һ�ķ���Ҳ�����Ʊ�NaClO4�����������Ʊ�HClO4��д�������ĵ缫��Ӧʽ ��

��1����ȴ���˵�Ŀ�� ��
��2��ͨ�뷴Ӧ��2��SO2������ ����Ӧ��2�з�����Ӧ�����ӷ���ʽΪ ��
��3��ѭ��ʹ�õ������� ��
��4������ͨ��������Һ�ķ����õ��������ԭ������� ��
��5��ͨ�����NaClO3ˮ��Һ�ķ���Ҳ�����Ʊ�NaClO4�����������Ʊ�HClO4��д�������ĵ缫��Ӧʽ ��
��1������NaHSO4���ܽ�Ȳ������NaHSO4���壨2�֣�
��2������������Ϊ��ԭ����ClO2��ԭΪNaClO2����2�֣�
2ClO2��SO2��4OH����2ClO2����SO42����2H2O����4�֣�
��3��H2SO4��2�֣�
��4��������ķе�Ƚϵͣ����״���Һ���ݳ�����2�֣�
��5��������Ӧʽ H2O+ClO3��-2e��= ClO4�� + 2H+����4�֣�
��2������������Ϊ��ԭ����ClO2��ԭΪNaClO2����2�֣�
2ClO2��SO2��4OH����2ClO2����SO42����2H2O����4�֣�
��3��H2SO4��2�֣�
��4��������ķе�Ƚϵͣ����״���Һ���ݳ�����2�֣�
��5��������Ӧʽ H2O+ClO3��-2e��= ClO4�� + 2H+����4�֣�
��

��ϰ��ϵ�д�
�����Ŀ

 ©����
©���� �����ᾧˮ��
�����ᾧˮ��


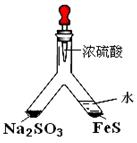
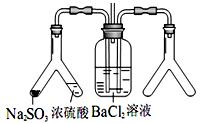
 ��Ŀ�ģ�̽�� SO2��BaCl2��Ӧ������������������������ͨ���Ȼ�����Һ����������������ͨ����һ���������Բ�����ɫ���������Ҳ� Y����Ӧ���õ�ҩƷ��____________��___________����Ҫʱ���Լ��ȣ����ó����Ļ�ѧʽΪ________________��
��Ŀ�ģ�̽�� SO2��BaCl2��Ӧ������������������������ͨ���Ȼ�����Һ����������������ͨ����һ���������Բ�����ɫ���������Ҳ� Y����Ӧ���õ�ҩƷ��____________��___________����Ҫʱ���Լ��ȣ����ó����Ļ�ѧʽΪ________________��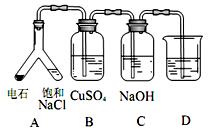
 ֤��ʯ������Ҫ�ɷ֣�D��ʢ��______________��
֤��ʯ������Ҫ�ɷ֣�D��ʢ��______________��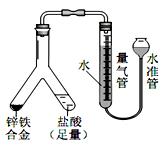

 ��C
��C H
H �ı�����ϵ�����ʵ������ͼ��ʾ��
�ı�����ϵ�����ʵ������ͼ��ʾ��
 D��Gװ�ü��ȣ��ڼ������װ�õ������ԣ����ų�װ���еĿ����ȡ���
D��Gװ�ü��ȣ��ڼ������װ�õ������ԣ����ų�װ���еĿ����ȡ��� l
l �������ѽ�Ĵ�����G����װ����ʡ�ԡ�
�������ѽ�Ĵ�����G����װ����ʡ�ԡ�



