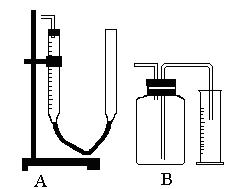
��Ŀ����
���� Y ��������������Ͽ��Խ�������ʵ�飨�̶�װ���ԣ����������ش��������⣺
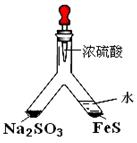
��1��ʵ��Ŀ�ģ���֤SO2�������ԡ�
����ͷ�ι���Ũ����ֱ���� Y�ܵ�����֧���У���֧�ܽ��洦ʵ������Ϊ__________________________________��
����������ˮ��Ŀ����____________________________��
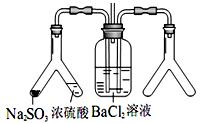
��2��ʵ ��Ŀ�ģ�̽�� SO2��BaCl2��Ӧ������������������������ͨ���Ȼ�����Һ����������������ͨ����һ���������Բ�����ɫ���������Ҳ� Y����Ӧ���õ�ҩƷ��____________��___________����Ҫʱ���Լ��ȣ����ó����Ļ�ѧʽΪ________________��
��Ŀ�ģ�̽�� SO2��BaCl2��Ӧ������������������������ͨ���Ȼ�����Һ����������������ͨ����һ���������Բ�����ɫ���������Ҳ� Y����Ӧ���õ�ҩƷ��____________��___________����Ҫʱ���Լ��ȣ����ó����Ļ�ѧʽΪ________________��
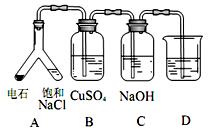
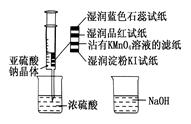
��3��ʵ��Ŀ�ģ�̽����ʯ���еijɷ֡�
��װ�� A�з�������Ҫ��ѧ��Ӧ����ʽΪ��___________________________��
�� Bװ���г��ֺ�ɫ������C װ�õ�����Ϊ__________��
��Ϊ�� ֤��ʯ������Ҫ�ɷ֣�D��ʢ��______________��
֤��ʯ������Ҫ�ɷ֣�D��ʢ��______________��
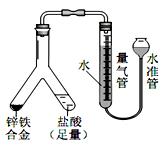
��4��ʵ��Ŀ�ģ�п���Ͻ����������IJⶨ
�ٶ�ȡ������������ʱ��������ˮ���е�Һ�������������Һ�棬Ӧ��ȡ�Ĵ�ʩ��___________________��
�����Ƶ�п���Ͻ������Ϊ 0.117g���������г�����Ϊ 1. 00mL��ĩ����Ϊ 45.80mL����Ͻ������ĺ���Ϊ_____%������2 λС����������������

��1��ʵ��Ŀ�ģ���֤SO2�������ԡ�
����ͷ�ι���Ũ����ֱ���� Y�ܵ�����֧���У���֧�ܽ��洦ʵ������Ϊ__________________________________��
����������ˮ��Ŀ����____________________________��

��2��ʵ
 ��Ŀ�ģ�̽�� SO2��BaCl2��Ӧ������������������������ͨ���Ȼ�����Һ����������������ͨ����һ���������Բ�����ɫ���������Ҳ� Y����Ӧ���õ�ҩƷ��____________��___________����Ҫʱ���Լ��ȣ����ó����Ļ�ѧʽΪ________________��
��Ŀ�ģ�̽�� SO2��BaCl2��Ӧ������������������������ͨ���Ȼ�����Һ����������������ͨ����һ���������Բ�����ɫ���������Ҳ� Y����Ӧ���õ�ҩƷ��____________��___________����Ҫʱ���Լ��ȣ����ó����Ļ�ѧʽΪ________________��
��3��ʵ��Ŀ�ģ�̽����ʯ���еijɷ֡�
��װ�� A�з�������Ҫ��ѧ��Ӧ����ʽΪ��___________________________��
�� Bװ���г��ֺ�ɫ������C װ�õ�����Ϊ__________��
����
 ֤��ʯ������Ҫ�ɷ֣�D��ʢ��______________��
֤��ʯ������Ҫ�ɷ֣�D��ʢ��______________��
��4��ʵ��Ŀ�ģ�п���Ͻ����������IJⶨ
�ٶ�ȡ������������ʱ��������ˮ���е�Һ�������������Һ�棬Ӧ��ȡ�Ĵ�ʩ��___________________��
�����Ƶ�п���Ͻ������Ϊ 0.117g���������г�����Ϊ 1. 00mL��ĩ����Ϊ 45.80mL����Ͻ������ĺ���Ϊ_____%������2 λС����������������
��12�֣�����ע��ÿ��1�֣�
��1���ܱ����е���ɫ�������ɣ�
ϡ��Ũ���ᣬ��ֹ���ⱻ����
��2��Ũ��ˮ����ʯ�ң����� NaOH����ʯ�ң��� BaSO3
[��ͭƬ��Ũ��� BaSO4 ���������ɣ�]
��3����CaC2 + 2H2O �� Ca(OH)2 + CH��CH������2�֣�
�ڳ�ȥ H2S��
��KMnO4��Һ����ˮ�����CCl4��Һ
��4����̧�ߣ����ƶ���ˮ��λ�ã�ʹˮ�ܡ���������Һ����ƽ��
��69.14%��2�֣�
��1���ܱ����е���ɫ�������ɣ�
ϡ��Ũ���ᣬ��ֹ���ⱻ����
��2��Ũ��ˮ����ʯ�ң����� NaOH����ʯ�ң��� BaSO3
[��ͭƬ��Ũ��� BaSO4 ���������ɣ�]
��3����CaC2 + 2H2O �� Ca(OH)2 + CH��CH������2�֣�
�ڳ�ȥ H2S��
��KMnO4��Һ����ˮ�����CCl4��Һ
��4����̧�ߣ����ƶ���ˮ��λ�ã�ʹˮ�ܡ���������Һ����ƽ��
��69.14%��2�֣�
��

��ϰ��ϵ�д�
 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�
�����Ŀ
 ����ش��������⣺
����ش��������⣺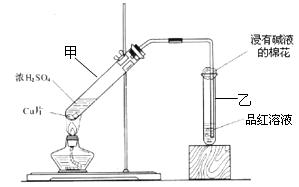
 L��Ba(OH)2��Һ�����ٲ�������ʱ��ǡ������40.00 mL Ba(OH)2��Һ������㣺
L��Ba(OH)2��Һ�����ٲ�������ʱ��ǡ������40.00 mL Ba(OH)2��Һ������㣺
 �����ƶ������Թ�Һ���У�ʹ֮�����ᷴӦ����Ӧ�����ӷ���ʽ�� ��
�����ƶ������Թ�Һ���У�ʹ֮�����ᷴӦ����Ӧ�����ӷ���ʽ�� ��