��Ŀ����
X��Y��Z��M��R��Q�Ƕ���������Ԫ�أ�������Ϣ���±���ʾ��
| X | Y | Z | M | R | Q | |
| ԭ�Ӱ뾶/nm | 0.186 | 0.074 | 0.099 | 0.143 | ||
| ��Ҫ���ϼ� | -4,+4 | -2 | -1,+7 | +3 | ||
| ���� | �����Ӻ������� | ���ǽ������ϵ����� | ��ɫ��Ӧ�ʻ�ɫ |
��Q�������������Z������������Ӧ��ˮ���ﷴӦ�����ӷ���ʽ��________��
��Y��R��ȣ��ǽ����Խ�ǿ����________����Ԫ�ط��ű�ʾ����������ʵ��֤����һ���۵���________��ѡ����ĸ��ţ���
A��������Y�ĵ��ʳʹ�̬��R�ĵ��ʳ���̬ B���ȶ���XR >YX4
C��Y��R�γɵĻ�������Y������
�ȸ��ݱ��������Ʋ⣬Y��ԭ�Ӱ뾶����С��Χ��________nm��r��Y����________nm ��
�����õ���ʽ��ʾZ2M���γɹ���________��
�������� IVA�� ͬλ�� Al2O3 + 2OH- = 2AlO2- + H2O Cl BC 0.143nm��r��Y����0.099nm�� ��
�ɱ������ݿ�֪��X��H��Y��Si��Z��Na��M��O����һ�������ۣ���R��Cl���ɰ뾶�ͻ��ϼ۵����ݿ���֪��Q��Al(�������ڵ�ԭ�Ӱ뾶����������0.1)��Q�������������Al2O3��Z������������Ӧ��ˮ������NaOH,��Ӧ�����ӷ���ʽΪAl2O3 + 2OH- = 2AlO2- + H2O����ͬ������Y����R���ұߣ�����Y�ķǽ����Խ�ǿ���ɸ��ݷǽ�����ǿ���жϹ�����֤������ѡBC,(4)����ͬ����ͬ����뾶��С�жϹ��ɿ�֪��0.143nm��r��Y����0.099nm��
�ɱ������ݿ�֪��X��H��Y��Si��Z��Na��M��O����һ�������ۣ���R��Cl���ɰ뾶�ͻ��ϼ۵����ݿ���֪��Q��Al(�������ڵ�ԭ�Ӱ뾶����������0.1)��Q�������������Al2O3��Z������������Ӧ��ˮ������NaOH,��Ӧ�����ӷ���ʽΪAl2O3 + 2OH- = 2AlO2- + H2O����ͬ������Y����R���ұߣ�����Y�ķǽ����Խ�ǿ���ɸ��ݷǽ�����ǿ���жϹ�����֤������ѡBC,(4)����ͬ����ͬ����뾶��С�жϹ��ɿ�֪��0.143nm��r��Y����0.099nm��

��ϰ��ϵ�д�
 ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
�����Ŀ
������Ԫ��X��Y��Z��M��R�����ڱ��е����λ����ͼ��ʾ������˵���в���ȷ���ǣ�������
| X | Y | ||||
| Z | M | R | |||
| A��Ԫ��X��Y�����γ��������ϵ���̬������ |
| B��ԭ�Ӱ뾶�Ĵ�С˳��Ϊ��r��Z����r��M����r��R�� |
| C��Ԫ�ص�����������Ӧˮ���������Rǿ��M |
| D������������Ԫ��R��Z�γɵĻ������ˮ��Һ���Եõ��û�����ľ��� |
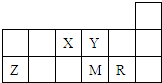 ������Ԫ��X��Y��Z��M��R�����ڱ��е����λ����ͼ��ʾ������˵����ȷ���ǣ�������
������Ԫ��X��Y��Z��M��R�����ڱ��е����λ����ͼ��ʾ������˵����ȷ���ǣ�������