��Ŀ����
2��
�����ѳ������л���Ӧ���ܼ���ʵ�����Ʊ������ѵķ�Ӧ����Ҫʵ��װ�����£�
2CH3CH2CH2CH2OH$?_{135��}^{Ũ����}$��CH3CH2CH2CH2��2O+H2O
��Ӧ��Ͳ���������������
| ��Է������� | �е�/�� | �ܶ�/��g•cm-3�� | ˮ���ܽ��� | |
| ������ | 74 | 117.2 | 0.8109 | �� |
| ������ | 130 | 142.0 | 0.7704 | �������� |
�ٽ�6mLŨ�����37g����������һ��˳�����ӵ�A�У����Ӽ�����ʯ��
�ڼ���A�з�ӦҺ��Ѹ��������135�棬ά�ַ�Ӧһ��ʱ�䣮
�����ᴿ��
�۴�A��Һ����ȴ���仺������ʢ��70mLˮ�ķ�Һ©���У����ã���Һ�ôֲ��
�ֲܴ���������40mLˮ��20mL NaOH��Һ��40mLˮϴ�ӣ���Һ�����Լ3g��ˮ�Ȼ��ƿ���������һ��ʱ�����ȥ�Ȼ��ƣ�
�ݽ������������Ĵֲ�����������ռ���֣��ô���������11g��
��ش�
��1���������Ũ�����������������˳��Ϊ�ȼ������������ټ���Ũ���ᣮ
��2������Aǰ�����ȴ�b���a����b��������B��ͨ��ˮ��
��3������۵�Ŀ���dz���ϴȥŨ���ᣬ���ã��ֲ���Ӧ�ӷ�Һ©�����ϣ���ϡ����¡����ڷ������
��4������������һ��ˮϴ��Ŀ��Ϊϴȥ�л����в�����NaOH���кͷ�Ӧ���ɵ��Σ�
��5��������У���������ʱӦ�ռ�d����ѡ����ĸ�����ҵ���֣�
a��100��b�� 117��
c�� 135��d��142��
��6����Ӧ�����л�۲쵽��ˮ�����ռ���Һ�����ʣ��ҷ�Ϊ�������㣬���ŷ�Ӧ�Ľ��У���ˮ����Һ��������������ʱ���ϲ�Һ�������֧���Զ�����A����ˮ�����ϲ�Һ�����Ҫ�ɷ�Ϊ���������²�Һ�����Ҫ�ɷ�Ϊˮ��
��7����ʵ���У������ѵIJ���Ϊ34%����������λ��Ч���֣�
���� ��1���������Ũ�����������������˳������Ũ�����ϡ�ͣ�
��2������ˮ��ˮ����������������෴���¿ڽ����Ͽڳ���
��3���ڷ�Ӧ������У�Ũ�������ܽ���ˮ�������Ѳ��ܣ�����������ˮ�����Բ���۵�Ŀ���dz���ϴȥŨ�����Һ©�������ú��ϲ�Һ����Ϊ�ܶȱ�ˮС�������Ѻ����������ӷ�Һ©�����Ͽڵ�����
��4������۽����һ��ˮϴ��Ŀ��Ϊϴȥ�л��������NaOH���кͷ�Ӧ���ɵ��Σ�
��5��������У����������Ŀ�����ռ������ѣ��������ѵķе�Ϊ142�棻
��6��Һ��������Ȼ�����������������������ת��ΪҺ�壬�������������ܶȱ�ˮС������ˮ�������������ϲ㣬ˮ���²㣻
��7��ʵ��ǰ37g�����������ʵ���Ϊ$\frac{37g}{74g/mol}$=0.5mol�����ݷ���ʽ���������������ѵ����ϵ��֪�����ѵ����۲������ʵ���Ϊ$\frac{0.5mol}{2}$=0.25mol����Ӧ�����ѵ�����Ϊ0.25mol��130g•mol-1=32.5g�������ѵIJ���Ϊ$\frac{11g}{32.5g}$%=34%��
��� �⣺��1���������Ũ�����������������˳������Ũ�����ϡ�ͣ�
�ʴ�Ϊ���ȼ������������ټ���Ũ���
��2������ˮ��ˮ����������������෴���¿ڽ����Ͽڳ���
�ʴ�Ϊ��b��
��3���ڷ�Ӧ������У�Ũ�������ܽ���ˮ�������Ѳ��ܣ�����������ˮ�����Բ���۵�Ŀ���dz���ϴȥŨ�����Һ©�������ú��ϲ�Һ����Ϊ�ܶȱ�ˮС�������Ѻ����������ӷ�Һ©�����Ͽڵ�����
�ʴ�Ϊ��Ũ����ϣ�
��4������۽����һ��ˮϴ��Ŀ��Ϊϴȥ�л��������NaOH���кͷ�Ӧ���ɵ��Σ�
�ʴ�Ϊ��ϴȥ�л����в�����NaOH���кͷ�Ӧ���ɵ��Σ�
��5��������У����������Ŀ�����ռ������������������ķе�Ϊ142�棬��Ӧ�ռ�142�����ҵ���֣�
�ʴ�Ϊ��d��
��6����ˮ�����ռ���Һ�����ʣ����������ܶȱ�ˮС������ˮ�����Ϊ�������㣬�ϲ�Ϊ���������²���Ҫ�ɷ�Ϊˮ��
�ʴ�Ϊ����������ˮ��
��7��ʵ��ǰ�ṩ��37g�����������ʵ���Ϊ$\frac{37g}{74g/mol}$=0.5mol�����ݷ���ʽ���������������ѵ����ϵ��֪�����ѵ����۲������ʵ���Ϊ$\frac{0.5mol}{2}$=0.25mol����Ӧ�����ѵ�����Ϊ0.25mol��130g•mol-1=32.5g�������ѵIJ���Ϊ$\frac{11g}{32.5g}$��100%=34%��
�ʴ�Ϊ��34%��
���� ���⿼��ʵ��Ļ��������������ķ���������ص㿼���Һ������������й����ʵ�����������Ͳ��ʵļ���ȣ���ע�������飬�е��Ѷȣ�

| A�� | �����μǡ��������һ������ͷ�ơ���ʮ���ǧ����ˡ����Ƽ��������У����е��Ʋ��ǽ��壬���ǽ��� | |
| B�� | ��ˮ䰡��о���Ƭ��-----�ǽ��������У��˸ǵ������ɺ�ҩҩ�����ǵ��ˣ���ʹ�õ��ɺ�ҩ����Ҫ�ɷݿ�����NaCN | |
| C�� | ���������塷�У���������������ϻ�ʱ·����Ȫ������������Ȫˮ��һ����˵��������������Ҫ�ɷ���CuSO4������ָ�̣�������Ȫˮ�ɽⶾ������Ȫˮ��Ҫ�ɷݿ������������� | |
| D�� | ����¥�Ρ����о�����䣺Ů����ˮ���Ĺ��⣬�����������Ĺ��⣮ˮ���Ӽ�ͨ�������϶���������������Ҫ�ɷ���̼��ƶ���Ӳ����仰���������Ů���Ը���� |
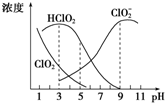 ����������һ�ָ�Ч��������Ư������Ҫ�����ġ�ֽ����Ư�ף��������ƣ�NaClO2������Һ�п�����ClO2��HClO2��ClO${\;}_{2}^{-}$��Cl-�ȣ�����HClO2��ClO2������Ư�����ã���ClO2���ж����壮���ⶨ��25��ʱ����ֺ�����pH�仯�����ͼ��ʾ��Cl-û�л������������з���������ǣ�������
����������һ�ָ�Ч��������Ư������Ҫ�����ġ�ֽ����Ư�ף��������ƣ�NaClO2������Һ�п�����ClO2��HClO2��ClO${\;}_{2}^{-}$��Cl-�ȣ�����HClO2��ClO2������Ư�����ã���ClO2���ж����壮���ⶨ��25��ʱ����ֺ�����pH�仯�����ͼ��ʾ��Cl-û�л������������з���������ǣ�������| A�� | ���������ڼ��������½��ȶ� | |
| B�� | 25��ʱ��HClO2�ĵ���ƽ�ⳣ������ԼΪKa=10-6 | |
| C�� | 25��ʱ��ͬŨ�ȵ�HClO2��Һ��NaClO2��Һ�������ϣ�����Cl-����������Һ����c��HClO2��+2c��H+���Tc��ClO${\;}_{2}^{-}$��+2c��OH-�� | |
| D�� | ʹ�ø�Ư�������pHΪ3 |
| A�� | ����ˮ��Ӧ��Na+2H2O�TNa++2OH-+H2�� | |
| B�� | ��������ͨ���Ȼ�������Һ��S2-+Fe2+�TFeS�� | |
| C�� | ������ͨ���Ȼ�������Һ��2Fe2++Cl2�T2Fe3++2Cl- | |
| D�� | ��������������������Һ��Al+2OH-�TAlO${\;}_{2}^{-}$+H2�� |
��CH3��CH2��3CH3
��CH3��CH2��4CH3
��C��CH3��4
�ܣ�CH3��2CHCH2CH3��
| A�� | �ڢ٢ܢ� | B�� | �ڢۢܢ� | C�� | �ۢܢ٢� | D�� | �٢ܢۢ� |
| A�� | ����ʹ�û�ʯȼ�� | B�� | ������չ�����Դ | ||
| C�� | ���ӹ����̴Ѹ߶� | D�� | ҹ���ŷŹ�ҵ���� |
 ����ӵ�ع㷺Ӧ�����ճ����Ӳ�Ʒ�У�Ҳ�ǵ綯����������ص���ѡ���������ϵ�ѡ�����������ӵ�ص����ܣ���������ﮣ�LiFePO4������߱����ԡ��߱���������ѭ���������߰�ȫ�ԡ��ͳɱ����������ŵ����Ϊ����Դ�³ʡ���
����ӵ�ع㷺Ӧ�����ճ����Ӳ�Ʒ�У�Ҳ�ǵ綯����������ص���ѡ���������ϵ�ѡ�����������ӵ�ص����ܣ���������ﮣ�LiFePO4������߱����ԡ��߱���������ѭ���������߰�ȫ�ԡ��ͳɱ����������ŵ����Ϊ����Դ�³ʡ���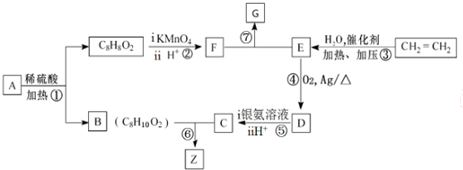
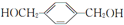 ������������������Ϊ�ǻ���
������������������Ϊ�ǻ��� ��
��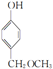 ��
��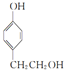 ��
��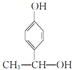 ��
��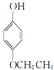 ��
��