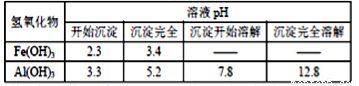
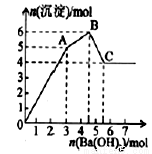
��Ŀ����
16�� �����£�ȡ20mLijŨ�ȵ�HCl��Ϊ����Һ����һ�����ʵ���Ũ�ȵ�NaOH��Һ���еζ�������������NaOH��Һ��Ϻ�����仯���Բ��ƣ����ζ���������Һ��pH�仯��ͼ��ʾ������������ȷ���ǣ�������
�����£�ȡ20mLijŨ�ȵ�HCl��Ϊ����Һ����һ�����ʵ���Ũ�ȵ�NaOH��Һ���еζ�������������NaOH��Һ��Ϻ�����仯���Բ��ƣ����ζ���������Һ��pH�仯��ͼ��ʾ������������ȷ���ǣ�������| A�� | ����HCl��Ũ����0.09mol•L��1��NaOH��ҺŨ��Ϊ0.03mol•L-1 | |
| B�� | ��B�㣬��Һ������Ũ�ȹ�ϵΪ��c��Na+����c��Cl-����c��H+����c��OH-�� | |
| C�� | A��B��C����ˮ�ĵ���̶ȴ�С����Ϊ��A��B��C | |
| D�� | �ζ�ǰ����ƿ�ô���Һ��ϴ������HClŨ��ƫ�� |
���� A��������������������Һ�����ʵ���Ũ�ȣ�Ȼ�����ͼ����������ʽ��������������������Һ��Ũ�ȣ�
B������B����������ж���Һ�и����ӵ�Ũ�ȴ�С��
C��A��BΪ������Һ��������ˮ�ĵ��룬������Ũ��Խ��ˮ�ĵ���̶�ԽС��C��Ϊ���ԣ�A��B��C��C��ˮ�ĵ���̶����
D����ƿ������ϴ�������´���Һ�����ʵ����ʵ���ƫ�����ĵı�Һ���ƫ�ⶨ���ƫ�ߣ�
��� �⣺A�����ͼ����HCl��Ũ����x mol•L��1��NaOH��ҺŨ��Ϊy mol•L��1���У�20��10��3��x=60��10��3��y��$\frac{0.02x-0.04y}{0.06}$=0.01����ã�x=0.09��y=0.03����A��ȷ��
B����B�㣬$\frac{2}{3}$�������кͣ���Һ�����ԣ�û�дﵽ��ȫ�кͣ�c��H+����c��OH������c��Cl������c��Na+������B����
C��A��B��C�����������μ�������ˮ�����һֱ�̶ȼ�С��ˮ�ĵ���̶ȴ�С����Ϊ��C��B��A����C����
D���ζ�ǰ����ƿ�ô���Һ��ϴ������n��H+��������n��NaOH��������HClŨ��ƫ�ߣ���D����
��ѡA��
���� ���⿼��������Ϻ���Һ�Ķ����жϼ���ҺpH�ļ��㡢ˮ�ĵ��뼰��Ӱ�����ء���Һ������Ũ�ȶ��ԱȽϵ�֪ʶ����Ŀ�Ѷ��еȣ�ע�����ձȽ���Һ������Ũ�ȴ�С�ķ�������ȷ��Һ����Զ�ˮ�ĵ���̶ȵ�Ӱ�죬����������ѧ�����Ӧ����ѧ֪ʶ��������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | �ؽᾧ���ᴿ������ʱ��Ϊ���������ྦྷ�壬����ҺҪ�ñ���ˮ�����ȴ | |
| B�� | ��Ȼ����������̬��Na��K��Si��Fe��������̬��S��O | |
| C�� | PM2.5��ָ������ֱ��С�ڻ����2.5�Ŀ����Ҳ��Ϊ��ϸ�������PM2.5�ڿ����в������γɽ��� | |
| D�� | ��ʯ����觡��轺����Ҫ�ɷ��Ƕ������� |
| A�� | ������ĵ���ʽ�� | |
| B�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | |
| C�� | S2-���ӵĽṹʾ��ͼ�� | |
| D�� | CS2����CO2����ֱ���ͷ��ӣ���CS2�ı���ģ��Ϊ |

| A�� | D���缫��Ӧ��O2+4e-+4H+=2H2O | B�� | E��ͨ��H2��C����O2�ų� | ||
| C�� | �׳���������ֻ�б�����ԭ | D�� | �����й�����14mol���� |
 ��1����ͭͬ���ڡ���̬ԭ��������������ͬ�Ĺ���Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽ[Ar]3d54s1��
��1����ͭͬ���ڡ���̬ԭ��������������ͬ�Ĺ���Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽ[Ar]3d54s1�� ���û�������BA3��Ӧ����B�ĵ��ʣ�д���÷�Ӧ�Ļ�ѧ��Ӧ����ʽ3HClO+2NH3=3HCl+N2+3H2O��
���û�������BA3��Ӧ����B�ĵ��ʣ�д���÷�Ӧ�Ļ�ѧ��Ӧ����ʽ3HClO+2NH3=3HCl+N2+3H2O��