��Ŀ����
9����ҵ������п��ɰ����Ҫ��ZnO��ZnFe2O4������������CaO��FeO��CuO��NiO���������ȡ����п��������ͼ��ʾ����֪�ö��Ե缫�������п��Һ������п���Գ����������ϣ������һ�㲻�����ף��ش��������⣺
��1�����ʱZnFe2O4�����������Σ��÷�Ӧ�Ļ�ѧ����ʽΪZnFe2O4+4H2SO4�TZnSO4+Fe2��SO4��3+4H2O��
��2�������������Ϊ��������һ���ǽ���Һ��������Fe2+�������ڶ����ǿ�����ҺpH��ʹFe3+ת��ΪFe��OH��3������
������Fe2+��ѡ�����������Լ�c��������ţ�
a��Fe2O3b����ˮ c��˫��ˮ d��ϡ����
��25��ʱ����Ũ�Ⱦ�Ϊ0.1mol/L��Ni2+��Fe3+�����ҺPH����Ϊ3����Һ����仯���Բ��ƣ�����ַ�Ӧ������Ũ�ȱ�$\frac{c��F{e}^{3+}��}{c��N{i}^{2+}��}$=4.0��10-10����֪25�棬Ksp[Fe�� OH��3]=4.0��10-38��Ksp[Ni�� OH��2]=1.0��10-17����
�۾��������ɵij����л�������Һ�е��������ʣ���Һ�е��������ʱ���ͬ������ԭ����Fe��OH��3���壨���������������ԣ�
��3����û�о�������������п���Ʊ�������Ӱ������ȡ��п��ͭ�����ʣ�
��4��д����ⷢ���Ļ�ѧ��Ӧ����ʽ2ZnSO4+2H2O$\frac{\underline{\;���\;}}{\;}$2H2SO4+2Zn+O2����
��5���������п���ѭ�����õ����ʳ�п���H2SO4��ZnSO4��
���� ��п��ɰ����Ҫ��ZnO��ZnFe2O4������������CaO��FeO��CuO��NiO������������������ӦCaO+H2SO4=CaSO4+H2O�����������ˮ����ZnFe2O4+8H+=Zn2++2Fe3++4H2O��ZnO+2H+=Zn2++H2O��FeO+2H+=Fe2++H2O��CuO+2H+=Cu2++H2O��NiO+2H+=Ni2++H2O������Һ�м���˫��ˮ��������Ӧ2Fe2++2H++H2O2=2 Fe3++2H2O��������Һ��pH��Fe3+ת��ΪFe��OH��3������������ҺpHʱ���������µ����ʣ�������ZnO��Ȼ������Һ�м���Zn�������û���ͭ������Ȼ����ˣ������õ�Zn���Դ˽����⣮
��� �⣺��1��������Ϣ�����ʱZnFe2O4�����������Σ��������ηֱ�Ϊ����п������������ZnFe2O4�����ᷴӦ��������п������������Ӧ�Ļ�ѧ����ʽΪ��ZnFe2O4+4H2SO4�TZnSO4+Fe2��SO4��3+4H2O��
�ʴ�Ϊ��ZnFe2O4+4H2SO4�TZnSO4+Fe2��SO4��3+4H2O��
��2���ټ��������������������������������ӣ�����������װ�ã�Ӧ����H2O2���ʴ�Ϊ��c��
��PH����Ϊ3����c��OH-��=10-11mol/L��Ksp[Fe�� OH��3]=4.0��10-38��Ksp[Ni�� OH��2]=1.0��10-17����֪c��Fe3+��=$\frac{4.0��1{0}^{-38}}{��1{0}^{-11}��^{3}}$��c��Ni2+��=$\frac{1.0��1{0}^{-17}}{��1{0}^{-11}��^{2}}$��
��$\frac{c��F{e}^{3+}��}{c��N{i}^{2+}��}$=4.0��10-10��
�ʴ�Ϊ��4.0��10-10��
���������ɵ�Fe��OH��3���壨���������������ԣ����Ծ��������ɵij����л�������Һ�е��������ʣ��ʴ�Ϊ��Fe��OH��3���壨���������������ԣ�
��3������п��ɰ�к���CaO��FeO��CuO��NiO���������Ӧ����Һ�д���ͭ���ӣ�û�о���������������ȡ��п�лẬ��ͭ�����ʣ�
�ʴ�Ϊ����ȡ��п��ͭ�����ʣ�
��4���������п��Һ�������ᡢп����������ⷽ��ʽΪ2ZnSO4+2H2O$\frac{\underline{\;���\;}}{\;}$2H2SO4+2Zn+O2����
�ʴ�Ϊ��2ZnSO4+2H2O$\frac{\underline{\;���\;}}{\;}$2H2SO4+2Zn+O2����
��5��ͨ����ȡ����п������ͼʾ����֪��������ѭ�����õ����ʳ�п�⣬�������ᡢ����п��
�ʴ�Ϊ��H2SO4��ZnSO4��
���� �����ۺϿ������ʵ��Ʊ��Լ����룬Ϊ�߿��������ͣ���Ŀ������ȡ����п�����̿��������ӷ���ʽ����ѧ����ʽ����д��֪ʶ��ע���������������Ϣ�������Ѷ��еȣ���ֿ����˷���������������

 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�| A�� | �춡�������ԭ�Ӷ�λ��ͬһƽ���� | |
| B�� | ������ˮ��Ӧ��ȡ�屽���ڼӳɷ�Ӧ | |
| C�� | ����������������ˮ������Ϊ������ | |
| D�� | �һ��������һ�������6�� |
| �������� | ��Ҫ��Դ | �Ի�����Ӱ�� | |
| A | ������̼ | ��ʯȼ�ϵ�ȼ�� | ���� |
| B | �������� | ����β�����ŷ� | �⻯ѧ���� |
| C | �������� | �����������ŷ� | ����ЧӦ |
| D | һ����̼ | ����ȩ��֬���������װ�β��� | �����ն� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| ������ | ȼ���� | ������ | ȼ���� |
| ���� | 891.0 | ������ | 2878.0 |
| ���� | 1560.8 | �춡�� | 2869.6 |
| ���� | 2221.5 | 2-������ | 3531.3 |
| A�� | ����ȼ�յ��Ȼ�ѧ����ʽΪ��2C2H6��g��+7O2��g��=4CO2��g��+6H2O��g����H=-1560.8 kJ/mol | |
| B�� | �ȶ��ԣ������飾�춡�� | |
| C�� | �������ȼ���ȴ���3531.3kJ/mol | |
| D�� | ��ͬ������������̼����������Խ��ȼ�շų�������Խ�� |
��������

| A�� | ԭ��Һ��һ��������Ag+��Al3+��Ba2+��SO42- | |
| B�� | ʵ�������ɳ��������ӷ���ʽ��SiO32-+2H+=H2SiO3�� | |
| C�� | ԭ��Һһ������K+��CO32-�����ܴ���NO3- | |
| D�� | ԭ��Һ������NO3-ʱ��c��K+��=0.8mol•L-1 |
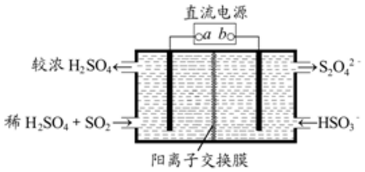 ������ͼ��ʾװ�ã��缫��Ϊ���Ե缫��������SO2�����������ų�����Һ������NO2������˵����ȷ���ǣ�������
������ͼ��ʾװ�ã��缫��Ϊ���Ե缫��������SO2�����������ų�����Һ������NO2������˵����ȷ���ǣ�������| A�� | aΪֱ����Դ�ĸ��� | |
| B�� | �� b�缫�����ĵ缫��ӦʽΪ��2HSO3-+2H++2e-�TS2O42-+2H2O | |
| C�� | �� a�����ĵ缫������ԭ��Ӧ�õ�SO42- | |
| D�� | ���ʱ��H+��������ͨ�������ӽ���Ĥ�������� |
| A�� | C2H4��C2H5OH��HOCH2CH2COOH | B�� | CH2O��C2H4O2��C6H12O6 | ||
| C�� | C6H6��C5H10��C8H6O2 | D�� | H2��CO��CH3OH |
| A�� | SiO2��MgO�۵�ߣ������������������� | |
| B�� | ��Ȼ���ǿ����������Դ�������ڹ�ҵ������ | |
| C�� | ������������������Ͻ��ÿ�ȼ���ֽ������װ | |
| D�� | ��ά�����ڶ��������ʣ��������������Ӫ������ |
| A�� | 18O3 | B�� | 2H217O2 | C�� | 14N16O2 | D�� | 14C16O2 |