��Ŀ����
������λͬѧ�ֱ��ò�ͬ�ķ�������100 mL 3.6 mol/L��ϡ���ᡣ
(1)������18 mol/L��Ũ����������Һ,��Ҫ�õ�Ũ��������Ϊ������������������
(2)��ѧ��:��ȡŨ����,С�ĵص���ʢ������ˮ���ձ���,�������,����ȴ�����º�ת�Ƶ�100 mL����ƿ��,��������ˮ���ձ�������ϴ��2��3��,ÿ��ϴ��ҺҲת�Ƶ�����ƿ��,Ȼ��С�ĵ�������ƿ����ˮ���̶��߶���,����ƿ��,�������µߵ�ҡ�ȡ�
�ٽ���Һת�Ƶ�����ƿ�е���ȷ������
������ʱ���ӿ̶���,��������ҺŨ����������(�ƫ����ƫС������Ӱ�족)��
��ϴ�Ӳ�����,��ϴ���ձ����ϴҺҲע������ƿ,��Ŀ������ ��
�۶��ݵ���ȷ�������� ��
���ý�ͷ�ι�������ƿ�м�ˮʱ,��С��Һ�泬���˿̶�,�����ķ�������������(�����)��
A.��������Һ��,ʹ��Һ����̶�������
BС�ļ�������ƿ,��������,ʹ��Һ����̶�������
C.���������һ������Ũ����
D.��������
(3)��ѧ��:��100 mL��Ͳ��ȡŨ����,��������С�ĵؼ�������ˮ,�������,����ȴ�����º�,�ټ���ˮ��100 mL�̶���,�ٽ�����ȡ�����Ϊ�˷��Ƿ���ȷ?������ȷ,ָ�����д���֮��:�� ��
(1)20.0 mL
(2)�ٽ���������������ƿ�̶�������,ʹ��Һ�ز�������������������ƿ�С�ƫС����ʹ������ȫת�Ƶ�����ƿ��
�ۼ�ˮ����̶���1��2 cmʱ,���ý�ͷ�ιܵμ�ˮ����Һ����̶������С���D
(3)����ȷ;��������Ͳ������Һ,���ܽ�ˮ���뵽Ũ������
����

���ǵ����Ϻ����ḻ��һ��Ԫ�أ�������(N2H4)�͵����ᶼ�ǵ�Ԫ�ص���Ҫ�⻯��ڹ�ũҵ�����������������ش����á�
(1)�ϳɰ����������Ĵ����������˹��̵���;�����Ի�ѧ��ҵ����Ҳ��������ҪӰ�졣
���ڹ̶�������ܱ������У��������»�ѧ��Ӧ��N2(g)��3H2(g)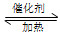 2NH3(g)����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3(g)����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
| T/K | 298 | 398 | 498 |
| ƽ�ⳣ��K | 4.1��106 | K1 | K2 |
��÷�Ӧ��ƽ�ⳣ���ı���ʽΪ________���ж�K1________K2(�����������������)��
�����и�����˵���÷�Ӧ�Ѵﵽƽ��״̬����________(����ĸ)��
a��������N2��H2��NH3��Ũ��֮��Ϊ1��3��2
b��v(N2)����3v(H2)��
c��������ѹǿ���ֲ���
d�����������ܶȱ��ֲ���
��һ���¶��£���1 L�ܱ������г���1 mol N2��3 mol H2������������Ӧ���������ݻ��㶨��10 min�ﵽƽ��ʱ������������ʵ���Ϊԭ����
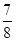 ����N2��ת����Ϊ________����NH3��Ũ�ȱ仯��ʾ�ù��̵ķ�Ӧ����Ϊ________��
����N2��ת����Ϊ________����NH3��Ũ�ȱ仯��ʾ�ù��̵ķ�Ӧ����Ϊ________��(2)�¿����ڻ��ȼ�ϡ���ҩԭ�ϵȡ�
���ڻ���ƽ�����װ����(N2H4)��Һ̬H2O2����֪0.4 molҺ̬N2H4������Һ̬H2O2��Ӧ��������̬N2����̬H2O���ų�256.6 kJ���������÷�Ӧ���Ȼ�ѧ����ʽΪ________________________________________________________________________��
��һ����ȼ�ϵ�صĹ���ԭ����ͼ��ʾ���õ�ع���ʱ�����ĵ缫��ӦʽΪ_____________________________________��

�ۼ�����������Һ̬��(N2H4)��ԭ����Cu(OH)2���Ʊ�����Cu2O��ͬʱ�ų�N2���÷�Ӧ�Ļ�ѧ����ʽ_______________________________________��
����������(HNO2)��Ӧ�����ɵ����ᣬ8.6 g��������ȫ�ֽ� �ɷų�6.72 L����(��״����)���������ķ���ʽΪ________��
 4FeO��CO2����
4FeO��CO2����