��Ŀ����
��1������������ˮ��Һ�ܵ��絫���ڷǵ���ʵ��� ������ţ����Ҵ� �ڰ��� ������ ���Ȼ��� ������ �ɱ� ��BaSO4 ����� ���������� ��CaO
��2��ijʵ����Ҫʹ��240ml 0.4mol/L CuSO4��Һ���õ������Ƹ�Ũ����Һ��Ҫʹ�õ�������������ƽ���ձ����������� �� ����Ҫ���� �˵����������Ƶ���ʧȥ���ֽᾧˮ�������Ƴ�����ҺŨ�� ������ƫ�ߡ�ƫ�ͻ���Ӱ�죩
��3���������й��Ŵ�����ٸ�Ŵ��ڻ��е�һ�����ϣ��仯ѧʽ��BaCuSi2O6���������������ʽ��ʾ����ɣ� ��
��1���ڢޣ���ѡ����ѡ����ѡ�����֣���2�֣�
��2��250ml ����ƿ��1��û��250ml���÷֣�����ͷ�ιܣ�1�֣���25.0��д25Ҳ���֣���2�֣���ƫ�ߣ�2�֣�
��3��BaO��CuO��2SiO2��2�֣�
���������������1���������ָ��ˮ��Һ���ۻ�״̬���ܵ���Ļ�������ǵ������ָ��ˮ��Һ���ۻ�״̬�¶����ܵ���Ļ��������ʰ����ᡢ��Ρ�ˮ�����ý�����������ʺͻ����Ȳ��ǵ����Ҳ���Ƿǵ���ʡ��ݴ��жϢ��Ȼ��� ������ ��BaSO4 ����� ���������� ��CaOΪ����ʣ����Ҵ� �ڰ��� �ɱ�Ϊ�ǵ���ʣ����Ҵ���ˮ��Һ�����磬��Ϊ�ڢޣ���2��һ�����ʵ���Ũ����Һ�����Ʋ��������У����㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ��������ݲ�������ѡ������������������õ�������ƽ��ҩ�ף��ܽ��õ��ձ��Ͳ���������Һ�õ�����ƿ��ʵ����û��240mL����ƿ��Ӧѡ��250mL����ƿ�������õ���ͷ�ιܣ�ȱ�ٵ�����Ϊ250ml ����ƿ����ͷ�ιܣ�����n=cv���������ͭ�����ʵ���������ͭ������ͭ��������ʵ�����ȣ��ٸ���m=nM������������ͭ�����������������ͭ���������Ϊm=0.25L��0.40mol?L-1��250g/mol=25.0g�������Ƶ���ʧȥ���ֽᾧˮ�������������ͭ������ƫ�����Ƴ�����ҺŨ��ƫ�ߣ���3���������й��Ŵ�����ٸ�Ŵ��ڻ��е�һ�����ϣ��仯ѧʽ��BaCuSi2O6����Ԫ��Ϊ+2�ۣ�ͭԪ��Ϊ+2�ۣ���Ԫ��Ϊ+4�ۣ���Ԫ��Ϊ��2�ۣ������������ʽ��ʾ�����Ϊ��BaO��CuO��2SiO2��
���㣺�������ʺͷǵ���ʵ��жϡ�һ�����ʵ���Ũ����Һ�����ơ����ϼ۹���ѧʽ����д��

 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�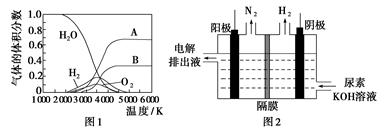
 ��C�봿���ڸ����µķ�Ӧ�ǹ�ҵ�Ʋ�������Ҫ��Ӧ֮һ��D����������Ԫ�ص�ԭ�Ӹ�����Ϊ3��4����������֮��Ϊ3��2������������Ϣ�ش��������⣺
��C�봿���ڸ����µķ�Ӧ�ǹ�ҵ�Ʋ�������Ҫ��Ӧ֮һ��D����������Ԫ�ص�ԭ�Ӹ�����Ϊ3��4����������֮��Ϊ3��2������������Ϣ�ش��������⣺