��Ŀ����
���õ���̬����������������������
����ͼ�ᳫ�������ж����������ڡ��ڼ��Լ�������ٳ������������������ı�ʶΪ ������ĸ����
�����������У�������������ˮɱ���������� ������ĸ����
a��Ư�� b������ c����������
�۹�ҵ��ˮ�账����������ŷţ����з�ˮ�����ķ����������� ������ĸ����
a�����кͷ���ȥ��ˮ�е���
b���û�������ȥ��ˮ�е��ؽ�������
c����������ȥ��ˮ�е�������
��ij������������Ⱦ��ָ��ΪS0278��NO2 43�������������153���õ�K����Ҫ��Ⱦ��Ϊ ��

����ͼ�ᳫ�������ж����������ڡ��ڼ��Լ�������ٳ������������������ı�ʶΪ
�����������У�������������ˮɱ����������
a��Ư�� b������ c����������
�۹�ҵ��ˮ�账����������ŷţ����з�ˮ�����ķ�����������
a�����кͷ���ȥ��ˮ�е���
b���û�������ȥ��ˮ�е��ؽ�������
c����������ȥ��ˮ�е�������
��ij������������Ⱦ��ָ��ΪS0278��NO2 43�������������153���õ�K����Ҫ��Ⱦ��Ϊ

���㣺���������������Ⱦ������,�ȡ��塢�⼰�仯������ۺ�Ӧ��,��ѧ�Լ��ķ���
ר�⣺
�������� Ϊ���������ı�ʶ��
Ϊ���������ı�ʶ��
�ں�HClO������������ӵ����ʡ��������Ⱦ�����������ˮ��ɱ�����������ж����ʲ�����������ˮ��ɱ���������Դ������
�۸���������ԭ�������ڳ����ࡢ�軯������������ڳ�ȥ��ˮ�е�����������������ڳ�ȥ��ˮ�е��ؽ������ӣ��кͷ������ڳ�ȥ��ˮ�е��
�ܸ�����Ҫ��Ⱦ��Ϊ��Ⱦָ�������жϣ�
 Ϊ���������ı�ʶ��
Ϊ���������ı�ʶ���ں�HClO������������ӵ����ʡ��������Ⱦ�����������ˮ��ɱ�����������ж����ʲ�����������ˮ��ɱ���������Դ������
�۸���������ԭ�������ڳ����ࡢ�軯������������ڳ�ȥ��ˮ�е�����������������ڳ�ȥ��ˮ�е��ؽ������ӣ��кͷ������ڳ�ȥ��ˮ�е��
�ܸ�����Ҫ��Ⱦ��Ϊ��Ⱦָ�������жϣ�
���
�⣺�� Ϊ���������ı�ʶ����ѡ��c��
Ϊ���������ı�ʶ����ѡ��c��
��Ư�۾���ǿ�����ԣ��������̼��Ӧ����HClO����Ư���ԣ��������Ⱦ���ǿ�����Ծ���Ư���ԣ�������û��ǿ�����ԣ�������ɱ�������Ĺ��ܣ�������������ˮ��ɱ����������ѡb��
��������ԭ�������ڳ����ࡢ�軯������������ڳ�ȥ��ˮ�е�����������������ڳ�ȥ��ˮ�е��ؽ������ӣ��кͷ������ڳ�ȥ��ˮ�е��ᣬ��ѡ��a��
����Ϊ��Ҫ��Ⱦ��Ϊ��Ⱦָ�����ģ����Ըõ�������Ҫ��Ⱦ��Ϊ�����������ʴ�Ϊ������������
 Ϊ���������ı�ʶ����ѡ��c��
Ϊ���������ı�ʶ����ѡ��c����Ư�۾���ǿ�����ԣ��������̼��Ӧ����HClO����Ư���ԣ��������Ⱦ���ǿ�����Ծ���Ư���ԣ�������û��ǿ�����ԣ�������ɱ�������Ĺ��ܣ�������������ˮ��ɱ����������ѡb��
��������ԭ�������ڳ����ࡢ�軯������������ڳ�ȥ��ˮ�е�����������������ڳ�ȥ��ˮ�е��ؽ������ӣ��кͷ������ڳ�ȥ��ˮ�е��ᣬ��ѡ��a��
����Ϊ��Ҫ��Ⱦ��Ϊ��Ⱦָ�����ģ����Ըõ�������Ҫ��Ⱦ��Ϊ�����������ʴ�Ϊ������������
������������Ҫ�����˳�������Ⱦ���������漰֪ʶ��϶࣬������ѧ֪ʶ������ɣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
֤��ij��Һ�к���Fe3+��ʵ�鷽���ǣ�������
| A���ȵμ���ˮ���ٵμ�KSCN��Һ���Ժ�ɫ |
| B���ȵ�KSCN��Һ�����Ժ�ɫ���ٵμ���ˮ���Ժ�ɫ |
| C���μ�NaOH��Һ���Ȳ�����ɫ������������ɫ�����ʺ��ɫ�� |
| D��ֻ��Ҫ�μ�KSCN��Һ |
��ѧ�뻷������ѧ������������أ������й�˵������ȷ���ǣ�������
| A����ʹ��������δ��������������� |
| B���ϳ���ά���ά�����л��߷��ӻ����� |
| C���⻯ѧ�������γ�������β���еĵ��������й� |
| D������ʹ�÷����Ͻ�֣��úϽ��۵㡢Ӳ�Ⱥ�ǿ�Ⱦ��ȴ����� |
��Ӧ4NH3��g��+5O2��g��?4NO��g��+6H2O��g����10L�ܱ������н��У�����Ӻ�ˮ���������ʵ���������0.45mol����˷�Ӧ��ƽ������
��X������Ӧ����������ʻ������������ʣ��ɱ�ʾΪ��������
. |
| v |
A��
| ||
B��
| ||
C��
| ||
D��
|
��ij����SO32-��SiO32-��CO32-��Br-��Na+����Һ�л���ͨ��Cl2ֱ�����������жԸ÷�Ӧ���̵��жϲ���ȷ���ǣ�������
| A���������̹�����2��������ԭ��Ӧ |
| B�����������ӵ�Ũ�ȶ������˱仯 |
| C����Һ����ɫ�����˱仯 |
| D����Ӧ�����м������������Ҳ�г������� |
���и�����������ɫ��Һ�����Դ������ǣ�������
| A��NH4+��NO3-��OH- |
| B��Ca2+��OH-��HCO3- |
| C��Na+��S2-��Cl- |
| D��Fe3+��Na+��Cl- |
���л�ѧ��Ӧ����������ԭ��Ӧ���ǣ�������
| A��2KOH+H2SO4=K2SO4+2H2O |
| B��2H2O2=2H2O+O2�� |
| C��CO2+2NaOH=NaCO3+H2O |
| D��NH3+HCl=NH4Cl |
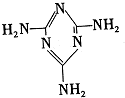 ���׳ƣ������������������з�Ӧ�ϳɣ�
���׳ƣ������������������з�Ӧ�ϳɣ�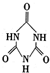 ��������֮����ͨ��
��������֮����ͨ��