��Ŀ����
��11�֣���ˮ�л�ѧ��Դ�����þ��зdz�������ǰ����
��1��Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ģ�����Ҫ�������£�
���Լ�������ѡ�� ������ţ���
A������ B��ʯ���� C���Ȼ�����Һ
�ڲ������������ ��
�۹�ҵ��ͨ��������ڵ�MgCl2�Ʊ�����Mg���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2����ͼ��ij����С����ʵ����ģ��Ӻ�ˮ����ȡ���ʵ��װ�á�
Aװ����ͨ��Cl2һ��ʱ���ͨ�ȿ�������Br2������Bװ�á�
��Aװ���з�����Ӧ����Br2�����ӷ���ʽΪ ��
�� Bװ���У�Br2��SO2��ԭ��Bװ���в�����������Ҫ�����ӵķ���Ϊ �� ��
��Cװ����NaOH��Һ�������� ��
��1����B �ڹ��� �� MgCl2 Mg + Cl2��
Mg + Cl2��
��2����Cl2+2Br��=== 2Cl��+Br2 �� SO Br��
Br��
�۷�ֹCl2��SO2��Br2�Ի�����Ⱦ(���һ�㼴��)
��������������Ţپ�����ѡ����ֻ��ʯ������MgSO4��Ӧ����Mg(OH)2������
��ͨ�����˽�Mg(OH)2�������������
�۵�����ڵ�MgCl2����Mg��Cl2��
�Ƣ�Aװ����Cl2��Br������������ԭ��Ӧ����Br2��Cl����
��Br2��SO2��ˮ��Һ�з�Ӧ����ʽΪBr2+ SO2+2H2O=H2SO4+2HBr�������������������ΪSO ��Br����
��Br����
�۸�װ�û����Cl2��SO2��Br2���ж����壬��NaOH��Һ��������Щ���壬��ֹ����Ⱦ������
���㣺����Ԫ�ؼ��仯����֪ʶ��ʵ���ۺϡ�

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д����н���ұ���ķ�Ӧԭ������ȷ����
A��2AlCl3(����)  2Al+3Cl2�� 2Al+3Cl2�� |
B��MgO+H2 Mg+H2O Mg+H2O |
C��ZnO+CO  Zn+CO2 Zn+CO2 |
D��2CuO  2Cu+O2�� 2Cu+O2�� |
��Դ��������������ᷢչ������أ����й�����Դ���������õ�˵���У�����Ϊ˵������ȷ���ǣ� ��
| A���������̫���� |
| B��������˿������÷��ܡ�ˮ�ܡ������ܡ���ϫ�� |
| C����������ȫ�����������ܡ����� |
| D����Դ����ͨ����ѧ��Ӧ��õ� |
[��ѧ��ѡ��2��ѧ�뼼��](15��)
��1�����й��ڹ�ҵ����˵����ȷ���� ��������ţ�
| A���ں����Ƽҵ�У����Ȼ�����Һ����ͨ������̼����ͨ���� |
| B�������Ṥҵ���ϳɰ���ҵ�����Ṥҵ�У��Բ���ѭ���������ԭ�������� |
| C�����ȼҵ�����۱����ӽ���Ĥ���������Һ������� |
| D����ҵ�ϲ��õ�������Ȼ����ķ�����ȡ������ |
��2���ҹ��涨����ˮ�������涨��������±���Ҫ��
| pH | Ca2+ ��Mg2+��Ũ�� | ϸ������ |
| 6.5��8.5 | �� 0.004 5 mol��L-1? | ��100����mL-1? |

��ԭˮ�к�Ca2+ ��Mg2+ ��HCO3-��Cl-�ȣ�����ʯ������Ca(OH)2�������������ɸ��ֽⷴӦ��д�����е����ӷ���ʽ��ֻҪ��д���������� �� ��
��FeSO4��7H2O�dz��õ����ۼ�������ˮ���������� ������ͨ�������̼��Ŀ���� �� ��
������A�������� ��ͨ��������Ca(ClO)2���A������������ ͬ��������Ϊ����A�Ĵ���Ʒ�����ţ���ѡ���ۣ���
a��ClO2 b��Ũ��ˮ c��K2FeO4 d��SO2
(1)���з�Ӧԭ�������Ϲ�ҵұ������ʵ���������(����)��
A��2HgO 2Hg+O2�� 2Hg+O2�� | B��Fe3O4+4CO 3Fe+4CO2 3Fe+4CO2 |
C��2MgO 2Mg+O2�� 2Mg+O2�� | D��2Ag2O 4Ag+O2�� 4Ag+O2�� |
�ҹ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ������ͼ��ʾ(ͼ��ijЩת�����輰������δ�г�):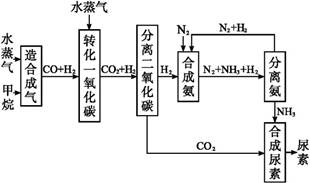
����д���пհ�:
(1)��֪0.5 mol�����0.5 molˮ������t�桢p kPaʱ,��ȫ��Ӧ����һ����̼������(�ϳ���),������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ����������������
(2)����������,��ҵ�Ϸ���H2��CO2�����ķ�������������
| A���������ͨ������������Һ,������Һ�м������� |
| B���������ѹ��ȴ,ʹCO2Һ�� |
| C��������ð�ˮϴ�� |
| D���������ͨ��ʯ�ҽ���,Ȼ��������չ��� |
(4)������������Դ����������߾���Ч��,Ҳ�Ƕ���ᡢ��ȫ���ฺ��ı���,�����߶κͼ�ͷ����ͼ�е���������������Դ�����
����˵���������(����)��
| A���ԷϾɽ�������ô��������ǻ��ա����� |
| B����������Ҫ������ʯ�ĸ�����ұ������������ |
| C�����ý�����ұ������ͨ�����������Һ |
| D���Ȼ�ԭ���л�ԭ���н�̿��һ����̼����������ý����� |